Expression and Values of Equilibrium Constant Using Partial
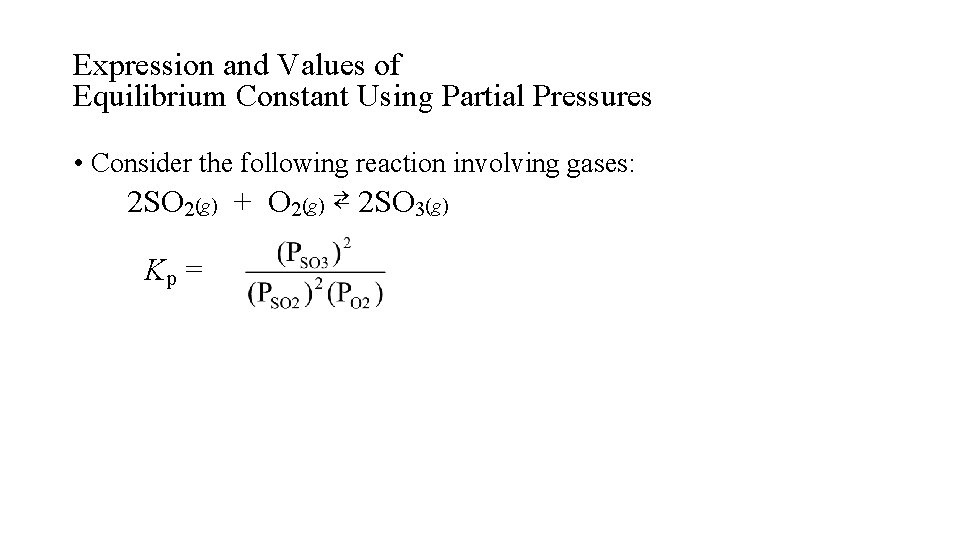
Expression and Values of Equilibrium Constant Using Partial Pressures • Consider the following reaction involving gases: 2 SO 2(g) + O 2(g) ⇄ 2 SO 3(g) Kp =
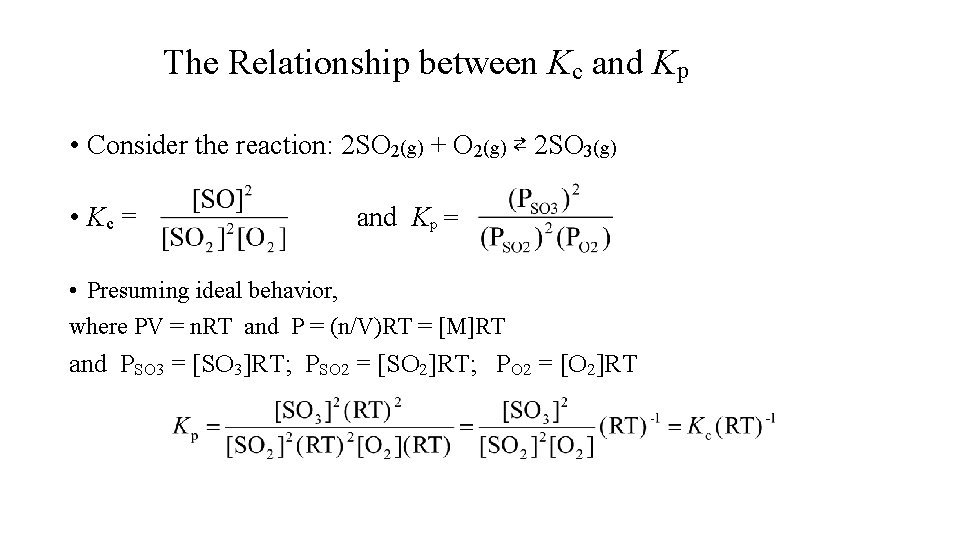
The Relationship between Kc and Kp • Consider the reaction: 2 SO 2(g) + O 2(g) ⇄ 2 SO 3(g) • Kc = and Kp = • Presuming ideal behavior, where PV = n. RT and P = (n/V)RT = [M]RT and PSO 3 = [SO 3]RT; PSO 2 = [SO 2]RT; PO 2 = [O 2]RT
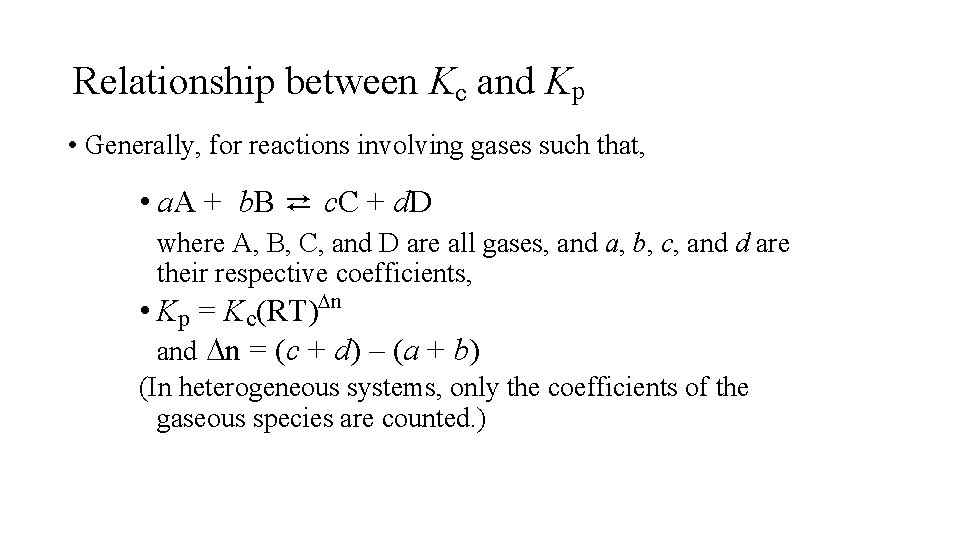
Relationship between Kc and Kp • Generally, for reactions involving gases such that, • a. A + b. B ⇄ c. C + d. D where A, B, C, and D are all gases, and a, b, c, and d are their respective coefficients, Dn • Kp = Kc(RT) and Dn = (c + d) – (a + b) (In heterogeneous systems, only the coefficients of the gaseous species are counted. )
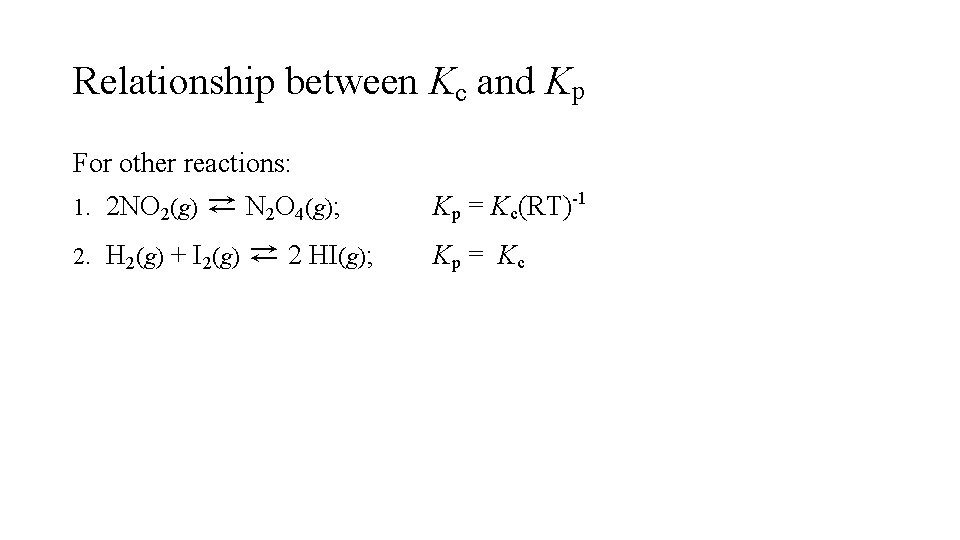
Relationship between Kc and Kp For other reactions: 1. 2 NO 2(g) ⇄ N 2 O 4(g); 2. H 2(g) + I 2(g) ⇄ 2 HI(g); Kp = Kc(RT)-1 Kp = Kc

Homogeneous & Heterogeneous Equilibria Homogeneous equilibria: CH 4(g) + H 2 O(g) ⇄ CO(g) + 3 H 2(g); Heterogeneous equilibria: Ca. CO 3(s) ⇄ Ca. O(s) + CO 2(g); HF(aq) + H 2 O(l) ⇄ H 3 O+(aq) + F-(aq);
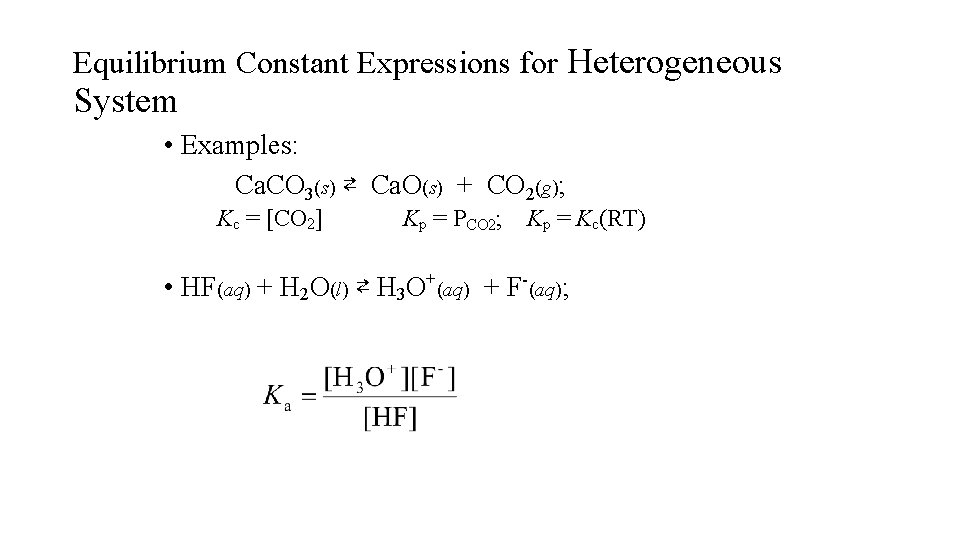
Equilibrium Constant Expressions for Heterogeneous System • Examples: Ca. CO 3(s) ⇄ Ca. O(s) + CO 2(g); Kc = [CO 2] Kp = PCO 2; Kp = Kc(RT) • HF(aq) + H 2 O(l) ⇄ H 3 O+(aq) + F-(aq);
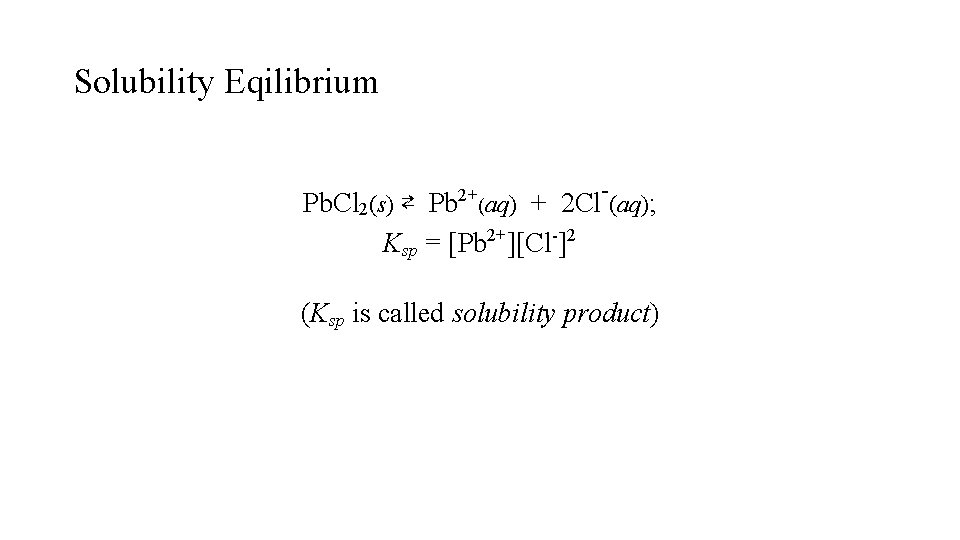
Solubility Eqilibrium - Pb. Cl 2(s) ⇄ Pb 2+(aq) + 2 Cl (aq); Ksp = [Pb 2+][Cl-]2 (Ksp is called solubility product)

Equilibrium Exercise #1 A flask is charged with 2. 50 atm of nitrogen dioxide and 1. 50 atm of dinitrogen tetroxide at 25 o. C and allowed to reach equilibrium. When equilibrium is established, the partial pressure of NO 2 has decreased by 1. 24 atm. (a) What are the partial pressures of NO 2 and N 2 O 4 at equilibrium? (b) Calculate Kp and Kc for following reaction at 25 o. C. 2 NO 2(g) ⇄ N 2 O 4(g)
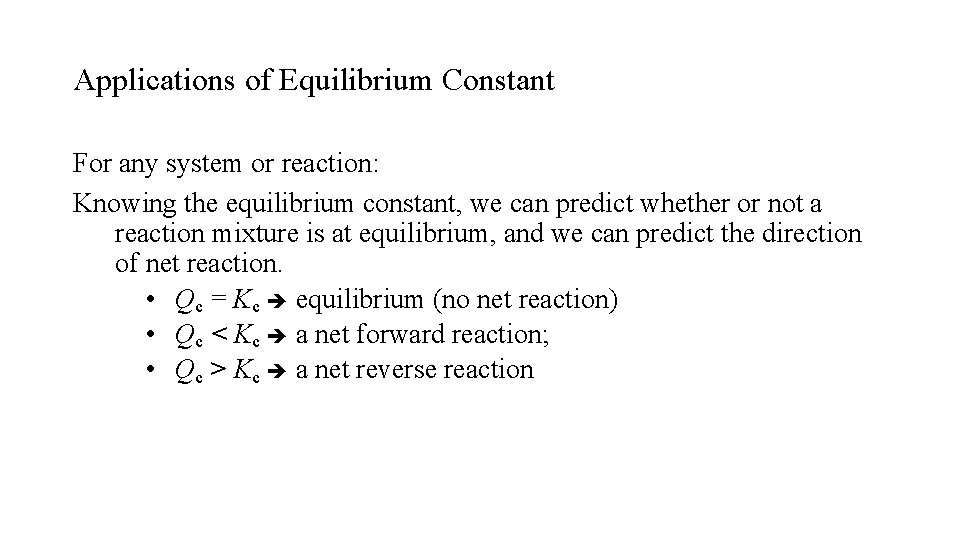
Applications of Equilibrium Constant For any system or reaction: Knowing the equilibrium constant, we can predict whether or not a reaction mixture is at equilibrium, and we can predict the direction of net reaction. • Qc = Kc equilibrium (no net reaction) • Qc < Kc a net forward reaction; • Qc > Kc a net reverse reaction
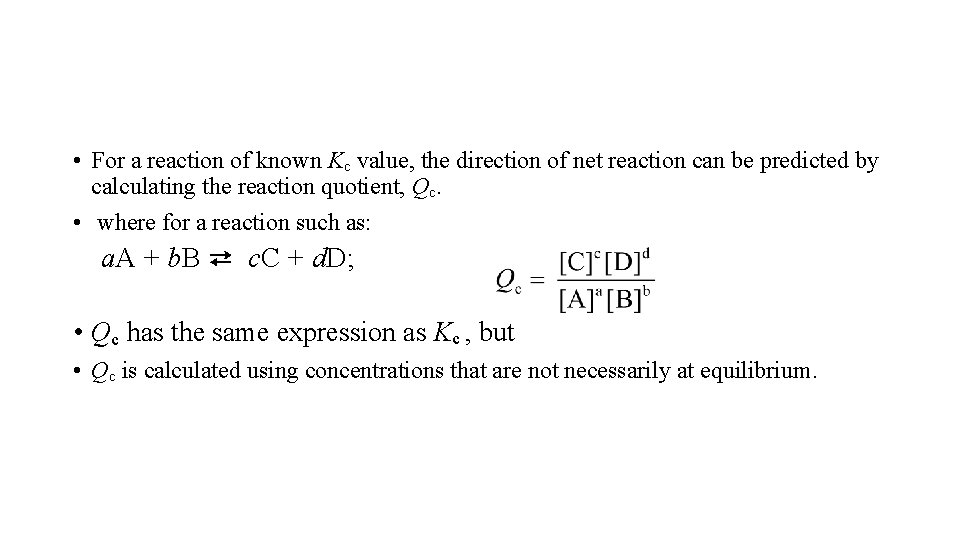
• For a reaction of known Kc value, the direction of net reaction can be predicted by calculating the reaction quotient, Qc. • where for a reaction such as: a. A + b. B ⇄ c. C + d. D; • Qc has the same expression as Kc , but • Qc is calculated using concentrations that are not necessarily at equilibrium.

Why is Equilibrium Constant Important? • Knowing Kc and the initial concentrations, we can determine the concentrations of components at equilibrium.
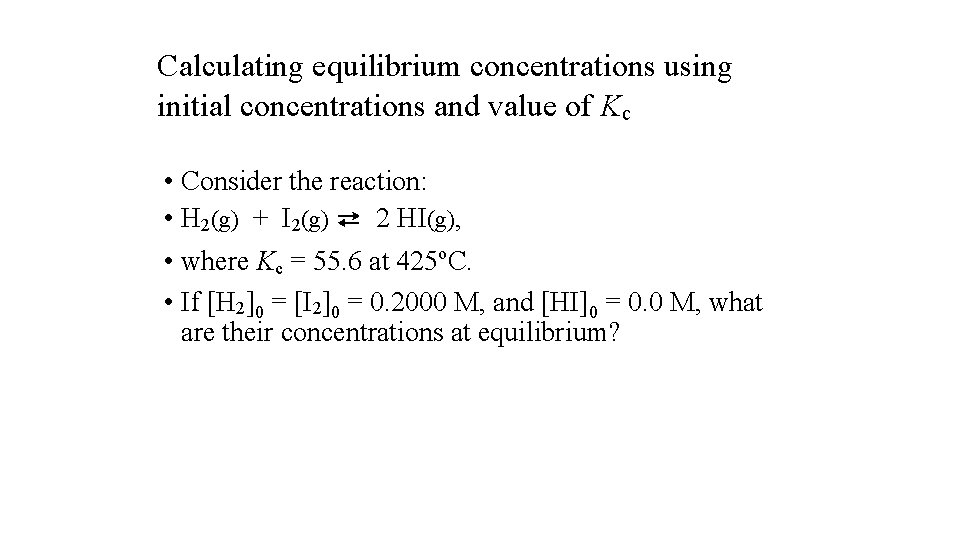
Calculating equilibrium concentrations using initial concentrations and value of Kc • Consider the reaction: • H 2(g) + I 2(g) ⇄ 2 HI(g), • where Kc = 55. 6 at 425 o. C. • If [H 2]0 = [I 2]0 = 0. 2000 M, and [HI]0 = 0. 0 M, what are their concentrations at equilibrium?
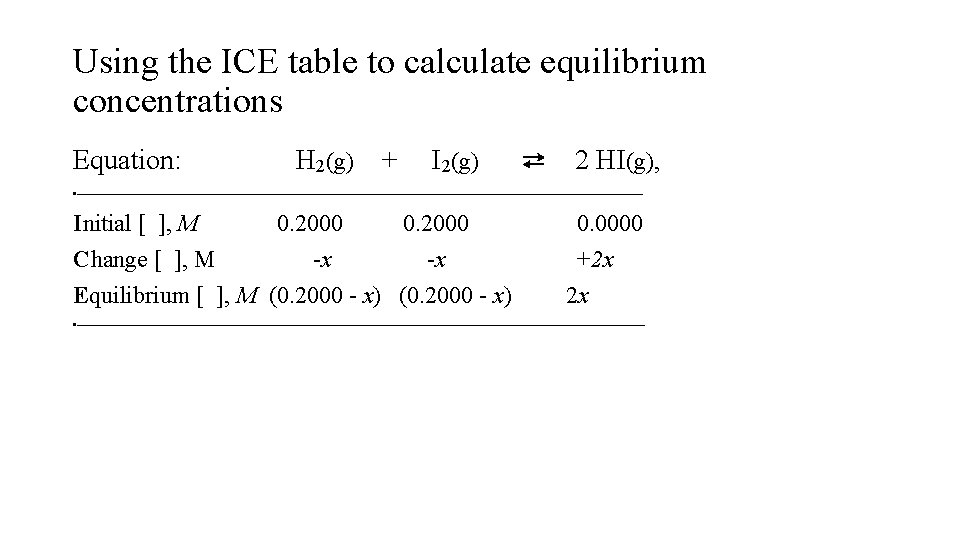
Using the ICE table to calculate equilibrium concentrations Equation: H 2(g) + I 2(g) ⇄ 2 HI(g), • Initial [ ], M 0. 2000 Change [ ], M -x -x Equilibrium [ ], M (0. 2000 - x) 0. 0000 +2 x 2 x •
- Slides: 13