Exposure in Draft SDTM IG 3 1 4
- Slides: 5

Exposure in Draft SDTM IG 3. 1. 4 Monika Kawohl Principal Statistical Programmer Accovion German Speaking CDISC UG Meeting, 25 -Sep-2012

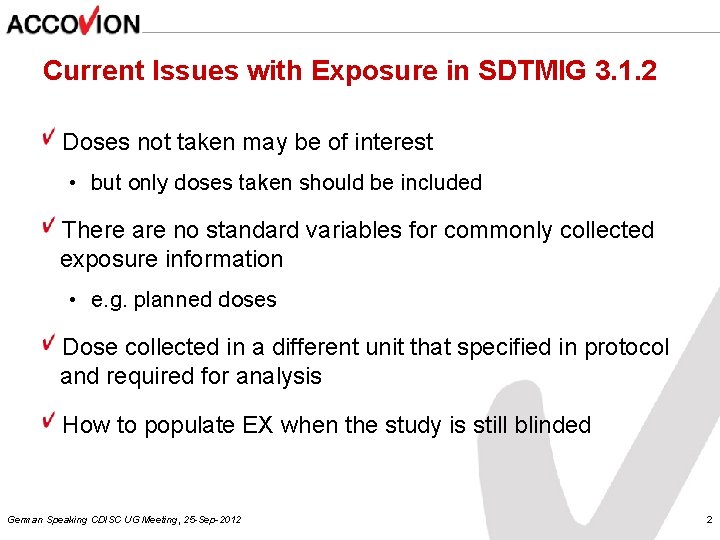
Current Issues with Exposure in SDTMIG 3. 1. 2 Doses not taken may be of interest • but only doses taken should be included There are no standard variables for commonly collected exposure information • e. g. planned doses Dose collected in a different unit that specified in protocol and required for analysis How to populate EX when the study is still blinded German Speaking CDISC UG Meeting, 25 -Sep-2012 2

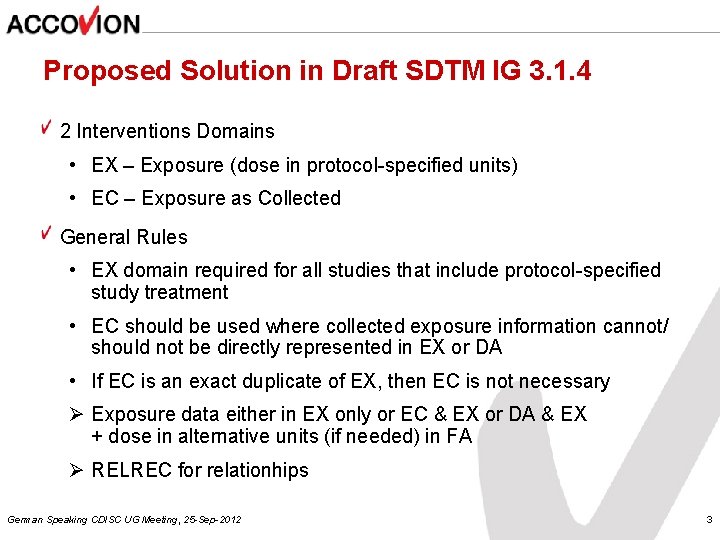
Proposed Solution in Draft SDTM IG 3. 1. 4 2 Interventions Domains • EX – Exposure (dose in protocol-specified units) • EC – Exposure as Collected General Rules • EX domain required for all studies that include protocol-specified study treatment • EC should be used where collected exposure information cannot/ should not be directly represented in EX or DA • If EC is an exact duplicate of EX, then EC is not necessary Ø Exposure data either in EX only or EC & EX or DA & EX + dose in alternative units (if needed) in FA Ø RELREC for relationhips German Speaking CDISC UG Meeting, 25 -Sep-2012 3

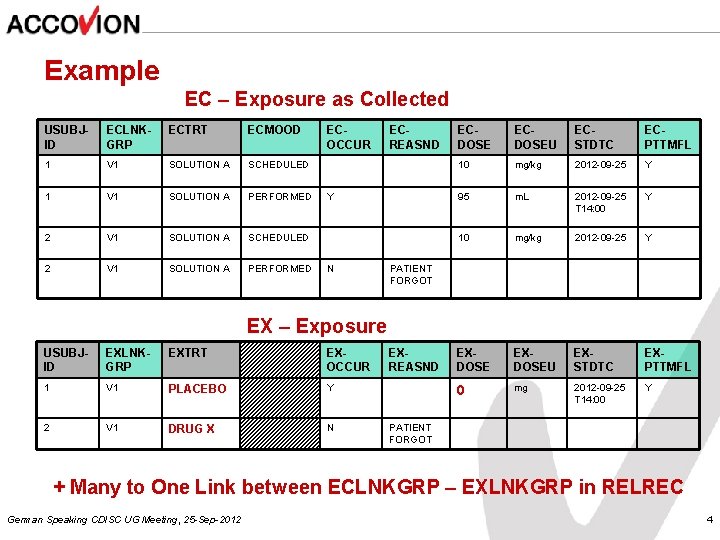
Example EC – Exposure as Collected USUBJID ECLNKGRP ECTRT ECMOOD 1 V 1 SOLUTION A SCHEDULED 1 V 1 SOLUTION A PERFORMED 2 V 1 SOLUTION A SCHEDULED 2 V 1 SOLUTION A PERFORMED ECOCCUR ECREASND Y ECDOSEU ECSTDTC ECPTTMFL 10 mg/kg 2012 -09 -25 Y 95 m. L 2012 -09 -25 T 14: 00 Y 10 mg/kg 2012 -09 -25 Y EXDOSEU EXSTDTC EXPTTMFL 0 mg 2012 -09 -25 T 14: 00 Y N PATIENT FORGOT EX – Exposure USUBJID EXLNKGRP EXTRT EXOCCUR 1 V 1 PLACEBO Y 2 V 1 DRUG X N EXREASND PATIENT FORGOT + Many to One Link between ECLNKGRP – EXLNKGRP in RELREC German Speaking CDISC UG Meeting, 25 -Sep-2012 4

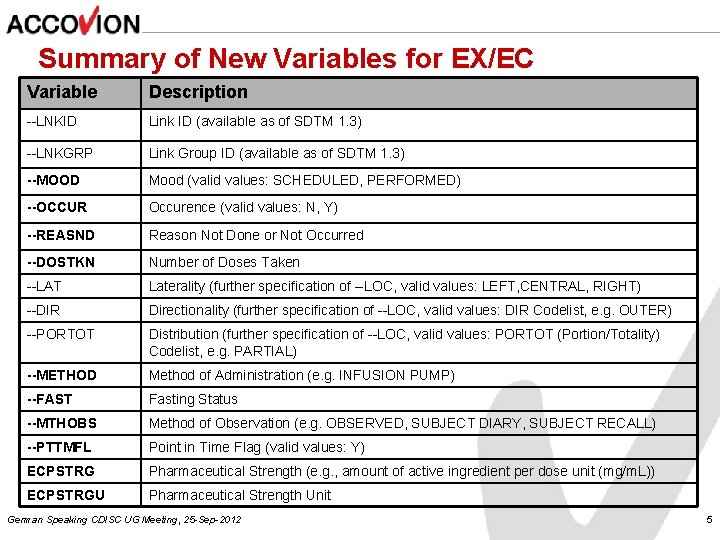
Summary of New Variables for EX/EC Variable Description --LNKID Link ID (available as of SDTM 1. 3) --LNKGRP Link Group ID (available as of SDTM 1. 3) --MOOD Mood (valid values: SCHEDULED, PERFORMED) --OCCUR Occurence (valid values: N, Y) --REASND Reason Not Done or Not Occurred --DOSTKN Number of Doses Taken --LAT Laterality (further specification of --LOC, valid values: LEFT, CENTRAL, RIGHT) --DIR Directionality (further specification of --LOC, valid values: DIR Codelist, e. g. OUTER) --PORTOT Distribution (further specification of --LOC, valid values: PORTOT (Portion/Totality) Codelist, e. g. PARTIAL) --METHOD Method of Administration (e. g. INFUSION PUMP) --FAST Fasting Status --MTHOBS Method of Observation (e. g. OBSERVED, SUBJECT DIARY, SUBJECT RECALL) --PTTMFL Point in Time Flag (valid values: Y) ECPSTRG Pharmaceutical Strength (e. g. , amount of active ingredient per dose unit (mg/m. L)) ECPSTRGU Pharmaceutical Strength Unit German Speaking CDISC UG Meeting, 25 -Sep-2012 5