Exploring the Periodic Table Standard SPS 1 Obtain
Exploring the Periodic Table
Standard SPS 1. Obtain, evaluate, and communicate information from the Periodic Table to explain the relative properties of elements based on patterns of atomic structure. b. Analyze and interpret data to determine trends of the following: Ø Number of valence electrons Ø Types of ions formed by main group elements Ø Location and properties of metals, nonmetals, and metalloids Ø Phases at room temperature c. Use the Periodic Table as a model to predict the above properties of main group elements.

Essential Questions • How is the periodic table arranged? What do elements on the same row have in common? What do elements in the same column have in common?

Essential Questions • How does its location on the • periodic table help you to predict the peoperties of an element?

Learning Targets • You should know the how the periodic table is arranged • You should know what elements on the same rows and columns have in common • You should know the trends of the periodic table including reactivity and atomic radius change

Dmitri Mendeleev • In the 1860’s he devised a periodic table where the elements were ordered by their atomic masses • Grouped elements on the basis of similar chemical properties. • Left blank spaces open to add new elements where he predicted they would occur.
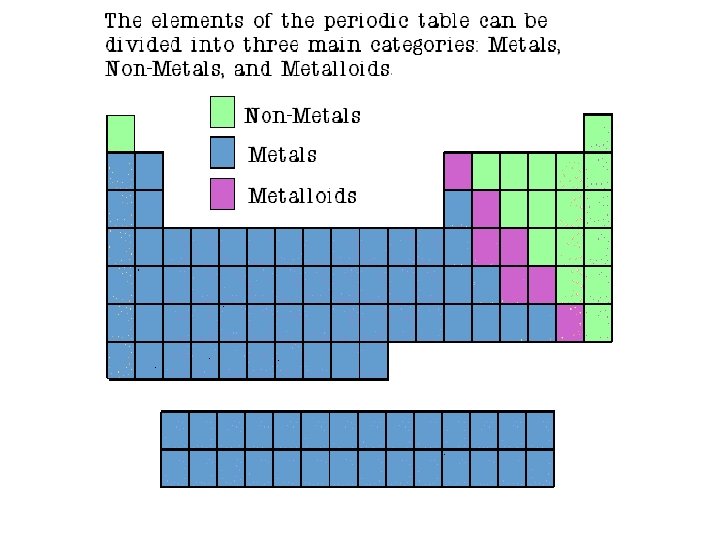
Properties of Metals • Metals are good conductors of heat and electricity. • Metals are shiny. • Metals are ductile (can be stretched into thin wires). • Metals are malleable (can be pounded into thin sheets). • A chemical property of metal is its reaction with water which results in corrosion.

Metal Facts • The metals are located on the left hand side of the periodic table. • Most of the elements on the periodic table are metals • Metals usually give up electrons to form cations and they have a positive charge. • Most metals are a solid at room temperature except mercury • The most reactive metal on the periodic table is francium

Properties of Non-Metals Sulfur • Non-metals are poor conductors of heat and electricity. • Non-metals are not ductile or malleable. • Solid non-metals are brittle and break easily. • They are dull. • Many non-metals are gases.

Nonmetal Facts • The nonmetals are located on the right hand side of the periodic table. Hydrogen is the only non-metal on the left hand side of the periodic table. • Nonmetals usually give up electrons to form cations and they have a positve charge. • Most nonmetals or solids or gases at room temperature. Bromine is the only nonmetal that is liquid at room temperature. • The most reactive nonmetal on the periodic table is fluorine

Properties of Metalloids • Metalloids (metal-like) have properties of both metals and non-metals. • They are solids that can be shiny or dull. • They conduct heat and electricity better than non-metals but not as well as metals. • They are ductile and malleable. Silicon
Metalloid Facts • Metalloids are found on the stair step on the periodic table. • Metalloids are also called semiconductors • The most well known semiconductor is silicon Silicon is used in computers and electronics • Metalloids can conduct electricity under certain conditions
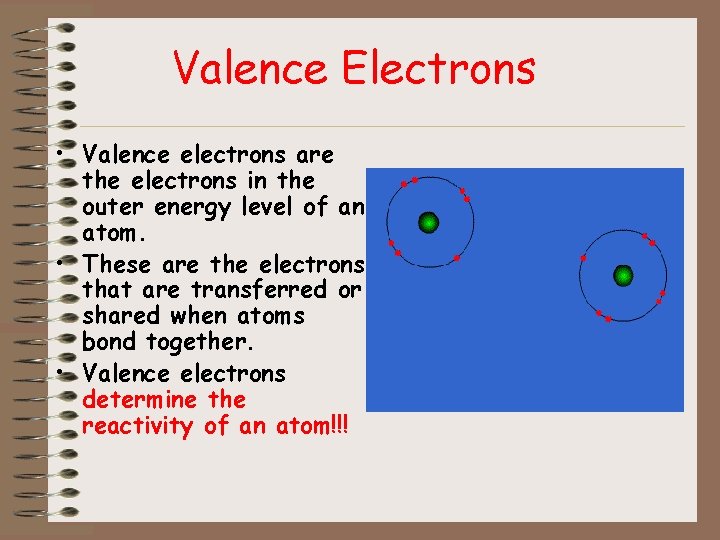
Valence Electrons • Valence electrons are the electrons in the outer energy level of an atom. • These are the electrons that are transferred or shared when atoms bond together. • Valence electrons determine the reactivity of an atom!!!
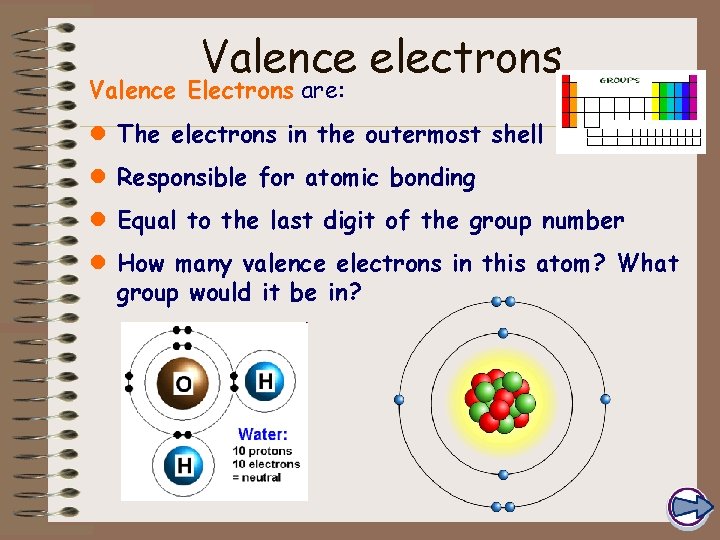
Valence electrons Valence Electrons are: l The electrons in the outermost shell l Responsible for atomic bonding l Equal to the last digit of the group number l How many valence electrons in this atom? What group would it be in?
The Periodic Table • Periodic means “repeating” pattern. • The periodic table groups similar elements together (think about sections in the grocery store). • Grouping makes it easier to predict the properties of an element.
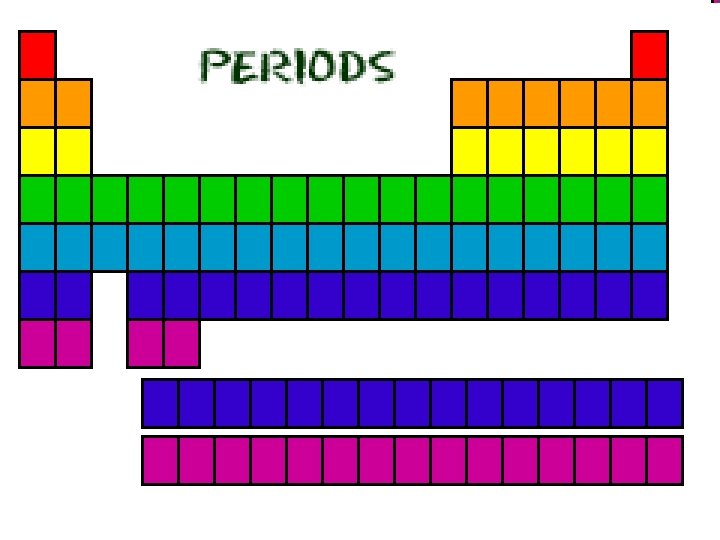
Periods Ø Periods: horizontal rows of elements Ø Just as the number of protons changes as you move from left to right across the periodic table, so does the number of electrons. Ø Remember that sentences are written in rows and end with a period Ø Elements in the same period have the same number of electron shells.
Groups Ø Groups: Vertical column of elements on the periodic table (18) Ø Remember that group is spelled group and groups go up and down Ø Elements in the same group have the same number of valence electrons Ø The valence electrons determine the reactivity of the element!!!!
Groups • Remember, valence electrons determine an element’s properties so all elements in the same group have similar properties. • What makes them different then? ? ?

Hydrogen is in the group with the alkalai metals because it has 1 valence electron H 1+ • Hydrogen belongs to a family of its own. • Hydrogen IS NOT A Metal • It is the only Non. Metal on the left side of the periodic table • Hydrogen is a diatomic (H 2), reactive gas. • Hydrogen was involved in the explosion of the Hindenberg. • Hydrogen is promising as an alternative fuel source for automobiles
ALKALI METALS Group 1 • Hydrogen is not a member, it is a non-metal • 1 electron in the outer shell, +1 charge • Soft and silvery metals • Very reactive, esp. with water • Conduct electricity • Alkali metals are never found as free elements in nature. They are always bonded with another element. Image:
ALKALINE EARTH METALS Group 2 • 2 electrons in the outer shell 2 • White and malleable • Reactive, but less than Alkali metals • Conduct electricity • They are never found uncombined in nature • +2 Charge
TRANSITION METALS Groups in the middle Ø Good conductors of heat and electricity. Ø Some are used for jewelry. Ø The transition metals are able to put up to 32 electrons in their second to last shell. Ø Can bond with many elements in a variety of shapes.
Halogens The “Salt Formers” Group 7 • 7 electrons in the outer shell • All are non-metals • Very reactive are often bonded with elements from Grp 1 • Halogen atoms only need to gain 1 electron to fill their outermost energy level. • They react with alkali metals to form salts.
Noble Gases Group 8 Ø Exist as gases Ø Non-metals Ø 8 electrons in the outer shell = Full Ø Helium (He) has only 2 electrons in the outer shell = Full Ø Not reactive with other elements Ø The family includes helium, neon, argon, krypton, xenon, and radon. Ø All the noble gases are found in small amounts in the earth's atmosphere. Ø Used in lighted “neon” signs
Rare Earth Metals Ø Some are Radioactive Ø The rare earths are silver, silvery -white, or gray metals. Ø The thirty rare earth elements are composed of the lanthanide and actinide series.
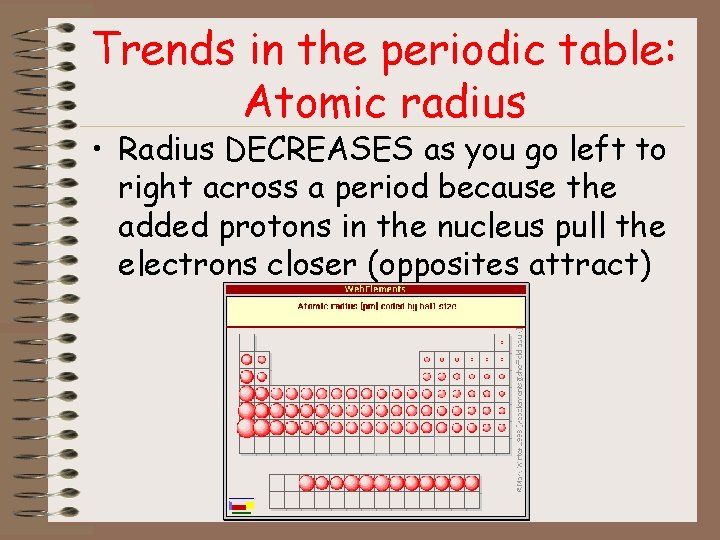
Trends in the periodic table: Atomic radius • Radius DECREASES as you go left to right across a period because the added protons in the nucleus pull the electrons closer (opposites attract)
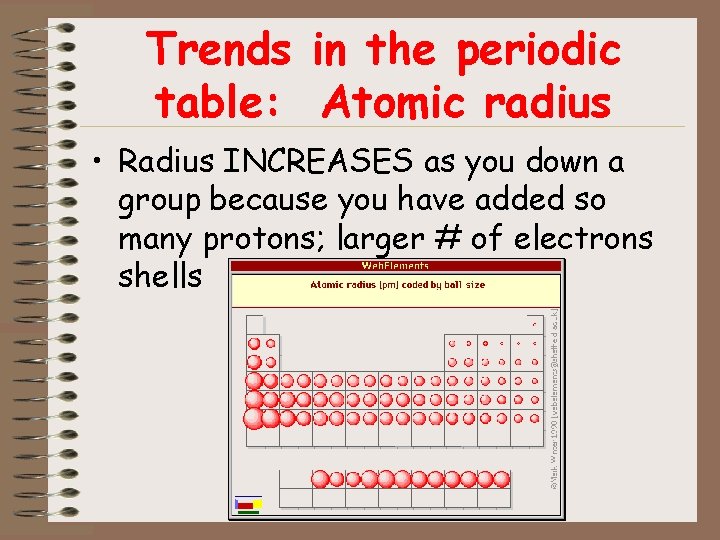
Trends in the periodic table: Atomic radius • Radius INCREASES as you down a group because you have added so many protons; larger # of electrons shells
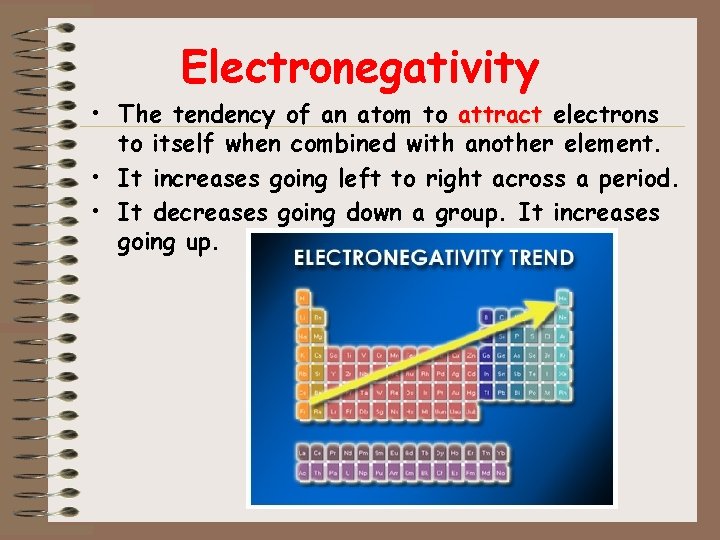
Electronegativity • The tendency of an atom to attract electrons to itself when combined with another element. • It increases going left to right across a period. • It decreases going down a group. It increases going up.
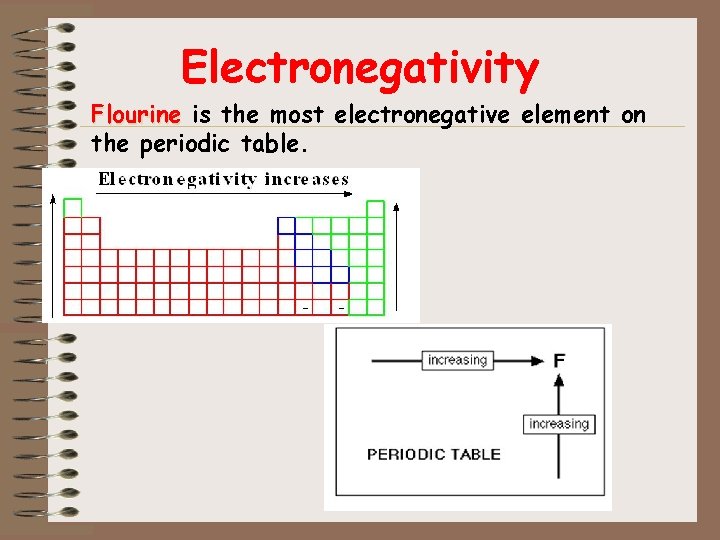
Electronegativity Flourine is the most electronegative element on the periodic table.

Chemical Reactivity • Reactivity is how easily an element will combine with other elements. – High reactivity = easier to combine • Elements combine with other elements by gaining, loosing or sharing electrons • Reactivity with metals increases as you go down and to the left Fracium • Reactivity on the right increases as you go up and to the right Flourine Except the Noble GASES!!!!!
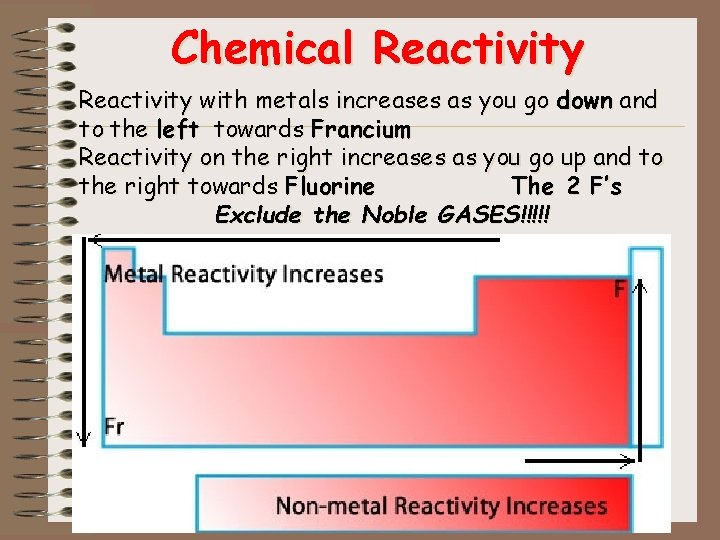
Chemical Reactivity with metals increases as you go down and to the left towards Francium Reactivity on the right increases as you go up and to the right towards Fluorine The 2 F’s Exclude the Noble GASES!!!!!
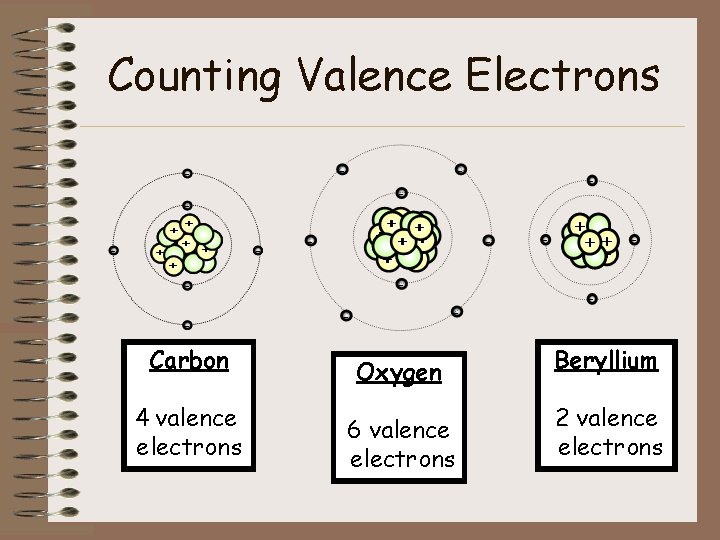
Counting Valence Electrons Carbon Oxygen Beryllium 4 valence electrons 6 valence electrons 2 valence electrons

Determining the Number of Valence Electrons by Using the Periodic Table *Atoms of elements in Groups 1 and 2 have the same number of valence electrons as their group number. *Atoms of elements in Group 3 -12 do not have a general rule relating their valence electrons to their group number. However, they typically have between 1 or 2 valence electrons. *Atoms of elements in Groups 13 -18 have 10 fewer valence electrons than their group number. (Exception - helium atoms have only 2 valence electrons, even though they are in group 18)
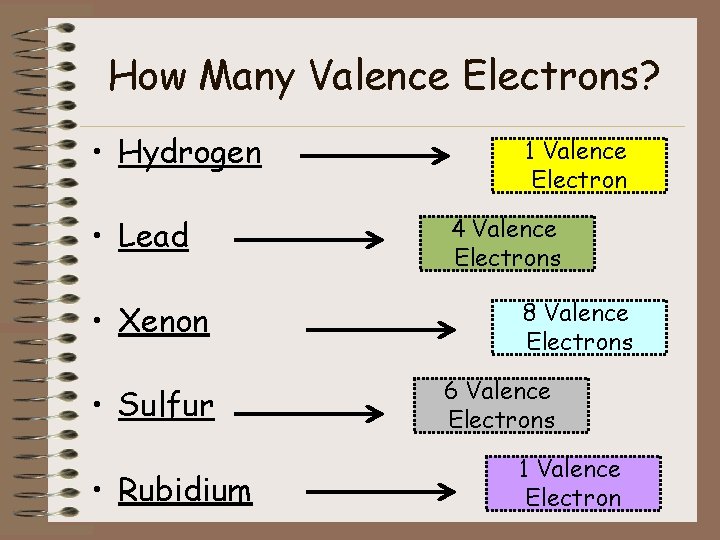
How Many Valence Electrons? • Hydrogen • Lead • Xenon • Sulfur • Rubidium 1 Valence Electron 4 Valence Electrons 8 Valence Electrons 6 Valence Electrons 1 Valence Electron
The Octet Rule • Atoms will combine to form compounds in order to reach eight electrons in their outer energy level. – Atoms with less than 4 electrons tend to lose electrons. – Atoms with more than 4 electrons tend to gain electrons. • Be aware that there are some exceptions! CONSIDER EIGHT A HAPPY NUMBER FOR ATOMS!
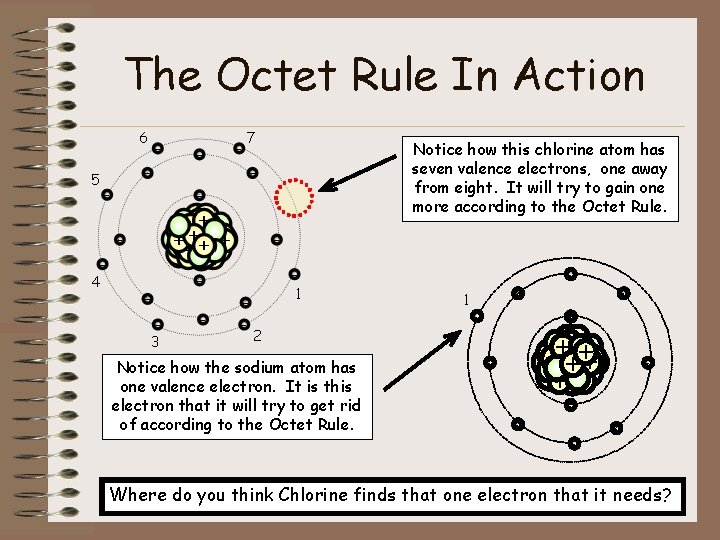
The Octet Rule In Action 6 7 Notice how this chlorine atom has seven valence electrons, one away from eight. It will try to gain one more according to the Octet Rule. 5 - 4 1 - 3 - 2 Notice how the sodium atom has one valence electron. It is this electron that it will try to get rid of according to the Octet Rule. - + ++ ++ + - - Where do you think Chlorine finds that one electron that it needs?

Octet Rule When atoms have a full outer valence shell they are
- Slides: 39