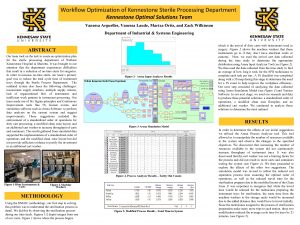
Explants Sterile pieces of a whole plant from













































- Slides: 66

Explants Sterile pieces of a whole plant from which cultures are generally initiated • Aerial plant parts are “cleaner” than underground parts • The smaller the explant the better the chances to overcome specific phytopathological problems (virus, microplasm, bacteria), but it decreases the survival rate • Inner tissues are less contaminated than outer ones • Comparable explants do not always react in a similar way , due to: influence of location on the mother plant, influence of juvenility status , influence of polarity

Types of explant Generally all plant cells can be used as an explant, however young and rapidly growing tissue (or tissue at an early stage of development) are preferred. Inoculum A subculture of plant material which is already in culture

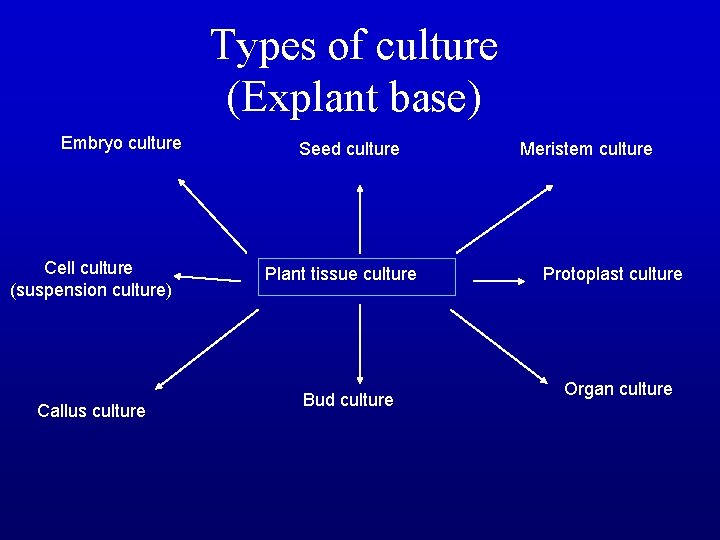
Types of culture (Explant base) Embryo culture Cell culture (suspension culture) Callus culture Seed culture Plant tissue culture Bud culture Meristem culture Protoplast culture Organ culture

Types of In vitro culture (explant based) ü Culture of intact plants (seed and seedling culture) ü Embryo culture (immature embryo culture) ü Organ culture ü Callus culture ü Cell suspension culture ü Protoplast culture

Seed culture ü Growing seed aseptically in vitro on artificial media ü Increasing efficiency of germination of seeds that are difficult to germinate in vivo ü it is possible to independent on asymbiotic germination. Production of clean seedlings for explants or meristem culture

Embryo culture ü Growing embryo aseptically in vitro on artificial nutrient media ü Overcoming seed dormancy and self-sterility of seeds ü Study embryo development

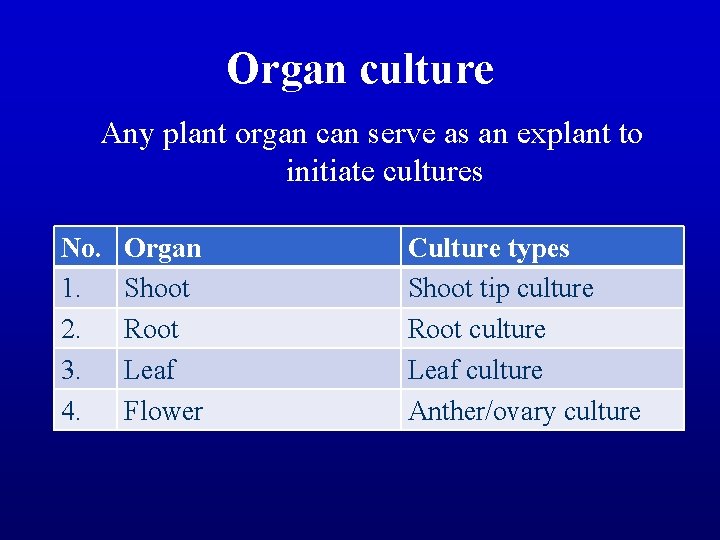
Organ culture Any plant organ can serve as an explant to initiate cultures No. 1. 2. 3. 4. Organ Shoot Root Leaf Flower Culture types Shoot tip culture Root culture Leaf culture Anther/ovary culture

Shoot apical meristem culture ü Production of virus free germplasm ü Mass production of desirable genotypes ü Facilitation of exchange between locations (production of clean material) ü Cryopreservation (cold storage) or in vitro conservation of germplasm

Root organ culture 1. Production of seedling from crop which multiply through root 2. Production of secondary metabolite

Ovary or ovule culture ü Production of haploid plants ü A common explant for the initiation of somatic embryogenic cultures ü Overcoming abortion of embryos of wide hybrids at very early stages of development due to incompatibility barriers ü In vitro fertilization for the production of distant hybrids avoiding style and stigmatic incompatibility that inhibits pollen germination and pollen tube growth

Anther and microspore culture ü Production of haploid plants ü Production of homozygous diploid lines through chromosome doubling, thus reducing the time required to produce inbred lines ü Uncovering mutations or recessive phenotypes

Sterilization Killing or excluding microorganisms or their spores with heat, filters, chemicals or other sterilants Tissue culture is an aseptic technique Aseptic technique: - Sterile - Free of pathogenic microorganisms - Free or freed from pathogenic microorganisms - Free from the living germs of disease and fermentation - Conditions established to exclude contaminants

Axenic culture ü ü ü Germfree Uncontaminated Free from germs or pathogenic organisms Free from other microorganism Containing only 1 organism A culture of an organism that is entirely free from all other contaminating organisms ü Not contaminated by or associated with any other living organism ü Pure cultures that are completely free of the presence of other organisms

Sterilization 1. Micro-organism contamination can over grow the plant culture resulting in culture death 2. Micro-organism contamination exhaust the nutrient media 3. Micro-organism can change in secondary metabolite structure or produce other compounds.

Source of contamination ü The explant or culture ü The vessels ü The media ü The instruments ü The environment where handling is taking place

Aseptic Techniques üChemical treatments • disinfectants, • antibiotics, • sublimat üPhysical treatments • heating: the most important disinfection method • electromagnetic radiation, • filtration • ultrasonic waves.

Disinfectans üThey penetrate into bacteria, üThey will denature bacterial protein, üThey decrease the activity of bacterial enzyme, üThey inhibit bacterial growth and metabolism, üThey damage the structure of cell membrane, üThey change membrane permeability.

Disinfectans – Liquid laundry bleach (Na. OCl at 5 -6% by vol) • Rinse thoroughly after treatment • Usually diluted 5 -20% v/v in water; 10% is most common – Calcium hypochlorite – Ca(OCl)2 • a powder; must be mixed up fresh each time – Ethanol (Et. OH) • • 95% used for disinfesting plant tissues Kills by dehydration Usually used at short time intervals (10 sec – 1 min) 70% used to disinfest work surfaces, worker hands – Isopropyl alcohol (rubbing alcohol) is sometimes recommended

Antibiotics üUsed only when necessary or when disinfestants are ineffective or impractical üIts use by incorporating in the media üCommon antibiotics are carbenicillin, cefotaxime, rifampicin, tetracycline, streptomycin üProblems with antibiotics • • tend to be selective resistance acquisition may obscure presence of microbes cell/tissue growth inhibition

An ideal antibiotics ü ü Broad-spectrum Did not induce resistance Selective toxicity, low side effects Preserve normal microbial flora 20 BC Yang

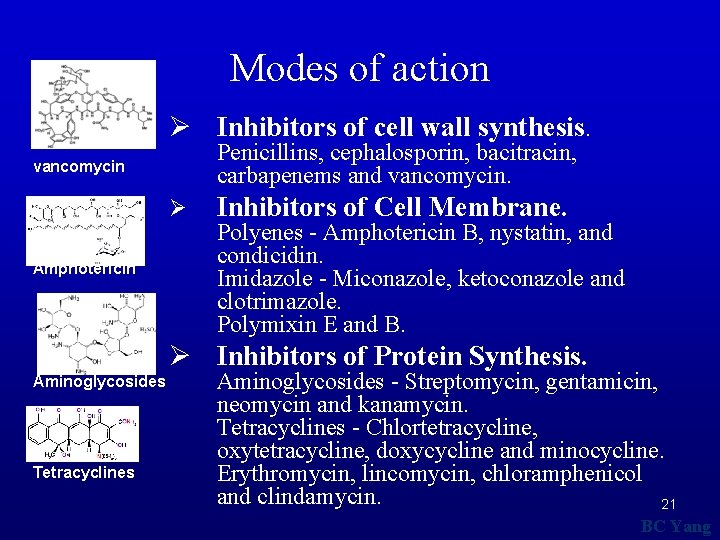
Modes of action Ø Inhibitors of cell wall synthesis. Penicillins, cephalosporin, bacitracin, carbapenems and vancomycin Ø Amphotericin Inhibitors of Cell Membrane. Polyenes - Amphotericin B, nystatin, and condicidin. Imidazole - Miconazole, ketoconazole and clotrimazole. Polymixin E and B. Ø Inhibitors of Protein Synthesis. Aminoglycosides Tetracyclines Aminoglycosides - Streptomycin, gentamicin, neomycin and kanamycin. Tetracyclines - Chlortetracycline, oxytetracycline, doxycycline and minocycline. Erythromycin, lincomycin, chloramphenicol and clindamycin. 21 BC Yang

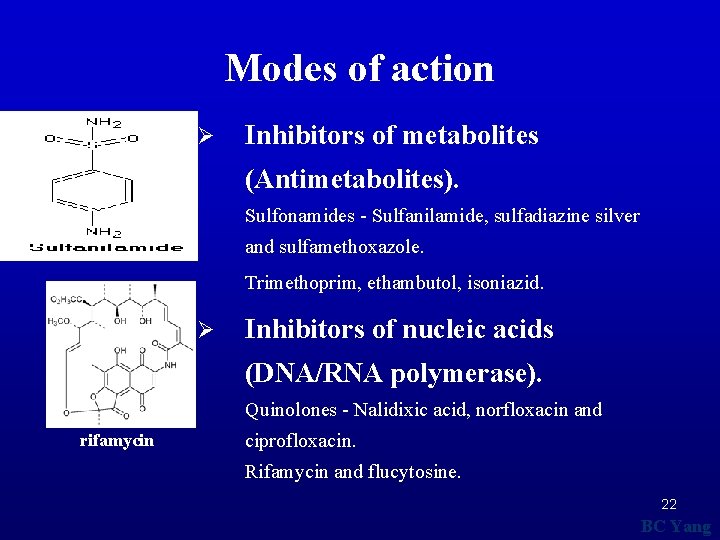
Modes of action Ø Inhibitors of metabolites (Antimetabolites). Sulfonamides - Sulfanilamide, sulfadiazine silver and sulfamethoxazole. Trimethoprim, ethambutol, isoniazid. Ø Inhibitors of nucleic acids (DNA/RNA polymerase). Quinolones - Nalidixic acid, norfloxacin and rifamycin ciprofloxacin. Rifamycin and flucytosine. 22 BC Yang

Sublimat (0. 1 - 1%) üIts activity based on Cl- üHeavy metal (Hg) denaturates proteins. üHg is toxic for the environment, therefore recuperate the Hg-solution after use and collect in a large container. ü Hg can be precipitated by adding ammonia to the solution, and siphoning the supernatant

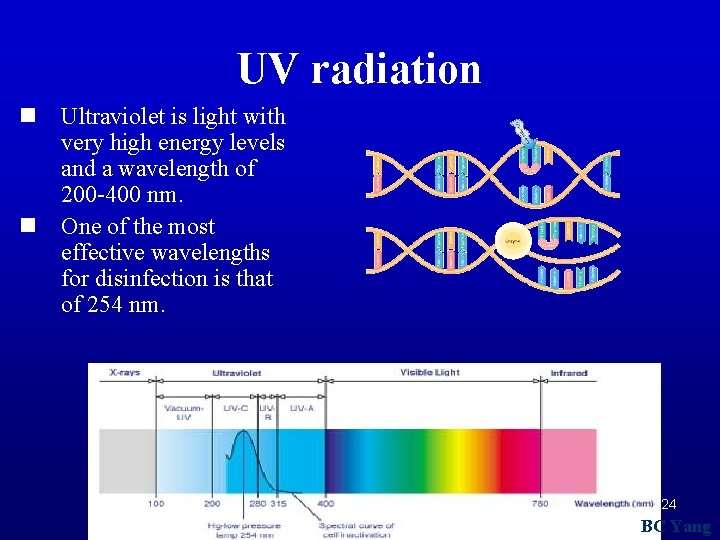
UV radiation n Ultraviolet is light with very high energy levels and a wavelength of 200 -400 nm. n One of the most effective wavelengths for disinfection is that of 254 nm. 24 BC Yang

Heating • Oven (dry heat) Suitable for tools, containers a 160°-180° C for 3 h • Microwaves (off the shelf) Useful for melting agar (but not gellan gum types of solidifying agents) Special pressurized containers are required for sterilizing in a microwave • Flaming or heating of tools Flaming – e. g. , 95% Et. OH in an alcohol burner is useful for sterilizing metal instruments Bacticinerators – heats metal tools in a hot ceramic core Heated glass beads

Heating • Autoclave Steam heat under pressure (It typically generates 15 lbs/in 2 and 250° F (1. 1 kg/cm 2 and 121° C)) It is faster and more effective For liquids (such as water, medium), autoclave time depends on liquid volume Recommended autoclaving times (sterilization time only): 250 ml requires 15 min 500 ml requires 20 min 1000 ml requires 25 min Excessive autoclaving can break down organics – a typical symptom is caramelized sucrose

Heating • Flaming or heating of tools Flaming – e. g. , 95% Et. OH in an alcohol burner is useful for sterilizing metal instruments Bacticinerators – heats metal tools in a hot ceramic core Heated glass beads

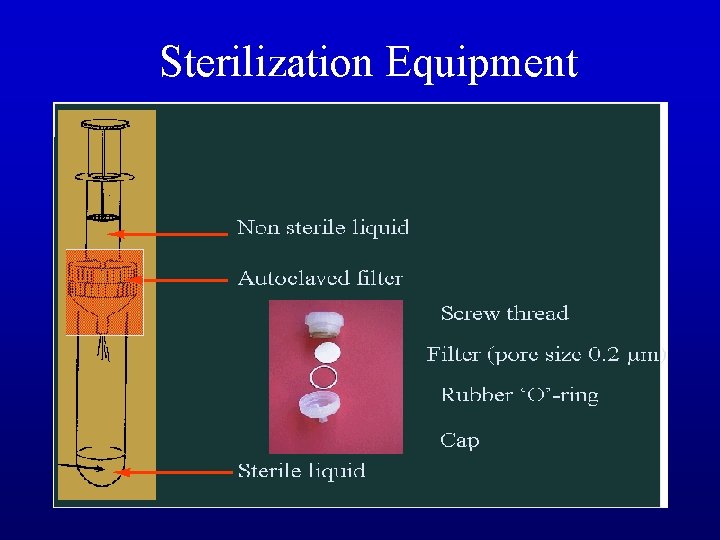
Filtration – Filtration of culture medium • Some medium ingredients are heat labile, e. g. , GA, IAA, all proteins, antibiotics • Most devices use a paper cellulose filter with small pore spaces (0. 22 µm) • Syringes used for small volumes, vacuum filtration for large volumes – Filtration of air • Transfer hoods usu. generate wind at 27 -30 linear m per min (or 90 -100 ft per min) • Too slow and air drops contaminants onto your work surface; too fast causes turbulence and excess filter wear • air "corridors" must be kept free of barriers to be effective

Sterilization Equipment

Sterilization Equipment sterilizing paper: dry heat sterilizing tools laminar flow cabinet

Sterilization Equipment

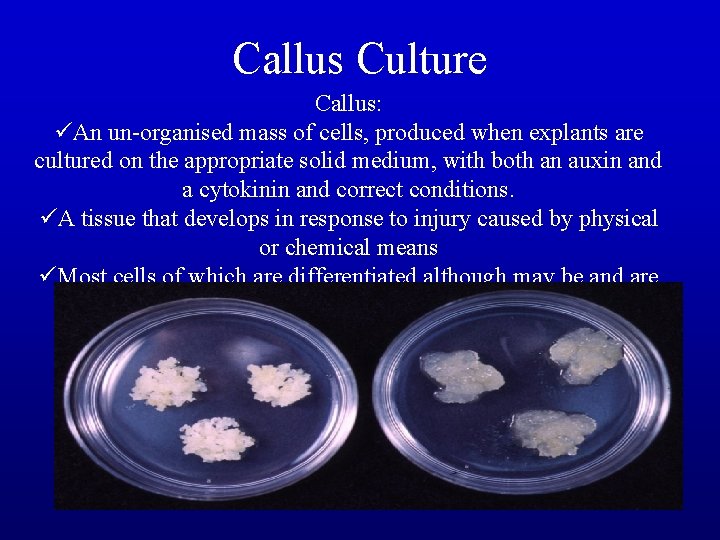
Callus Culture Callus: üAn un-organised mass of cells, produced when explants are cultured on the appropriate solid medium, with both an auxin and a cytokinin and correct conditions. üA tissue that develops in response to injury caused by physical or chemical means üMost cells of which are differentiated although may be and are often highly unorganized within the tissue

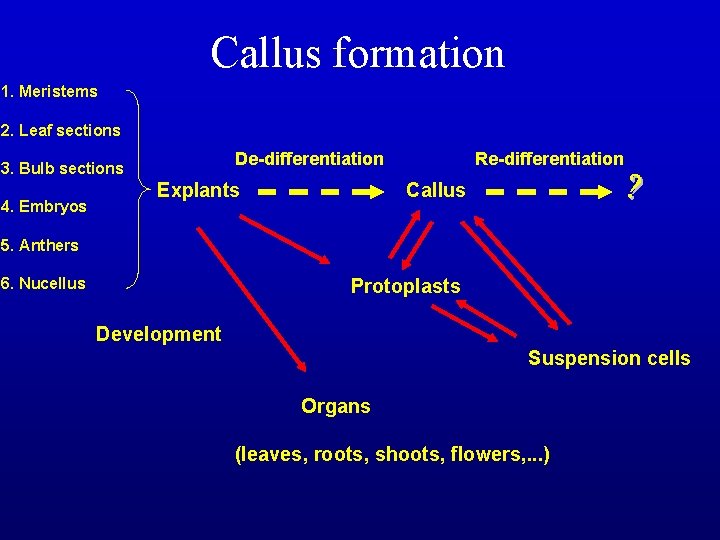
Callus formation 1. Meristems 2. Leaf sections 3. Bulb sections 4. Embryos 5. Anthers 6. Nucellus De-differentiation Explants Re-differentiation Callus Protoplasts Development Suspension cells Organs (leaves, roots, shoots, flowers, . . . )

Callus formation Stimuli : In vivo : wound, microorganisms, insect feeding In vitro : Phytohormones 1. Auxin 2. Cytokinin 3. Auxin and cytokinin 4. Complex natural extracts

Callus • During callus formation there is some degree of dedifferentiation both in morphology and metabolism, resulting in the lose the ability to photosynthesis. • Callus cultures may be compact or friable. üCompact callus shows densely aggregated cells üFriable callus shows loosely associated cells and the callus becomes soft and breaks apart easily. • Habituation: The lose of the requirement for auxin and/or cytokinin by the culture during long-term culture. •

Cell-suspension cultures ü When friable callus is placed into the appropriate liquid medium and agitated, single cells and/or small clumps of cells are released into the medium and continue to grow and divide, producing a cell-suspension culture. ü The inoculum used to initiate cell suspension culture should neither be too small to affect cells numbers nor too large too allow the build up of toxic products or stressed cells to lethal levels. ü When callus pieces are agitated in a liquid medium, they tend to break up.

Cell suspension culture ü Suspensions are much easier to bulk up than callus since there is no manual transfer or solid support ü Cell suspension culture techniques are very important for plant biotransformation and plant genetic engineering.

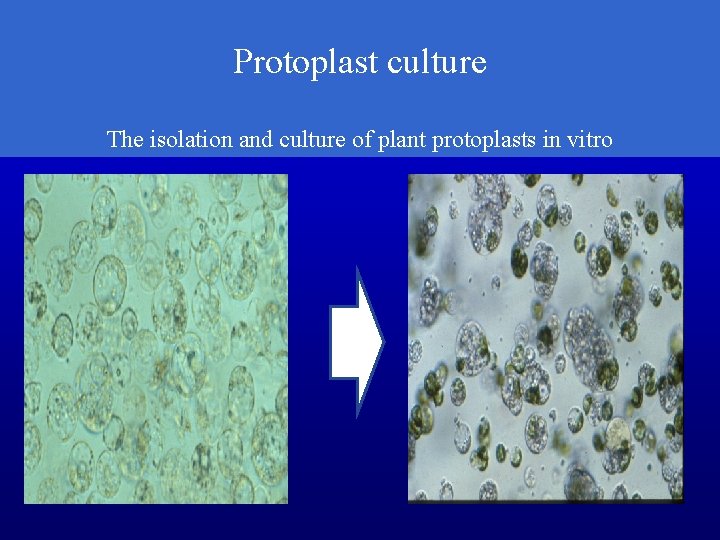
Protoplast culture The isolation and culture of plant protoplasts in vitro

Protoplast The living material of a plant or bacterial cell, including the protoplasm and plasma membrane after the cell wall has been removed.

Plant Regeneration Pathways Ø Existing Meristems (Microcutting) Uses meristematic cells to regenerate whole plant. Ø Organogenesis Relies on the production of organs either directly from an explant or callus structure Ø Somatic Embryogenesis Embryo-like structures which can develop into whole plants in a way that is similar to zygotic embryos are formed from somatic cells (Source: Victor. et al. , 2004)

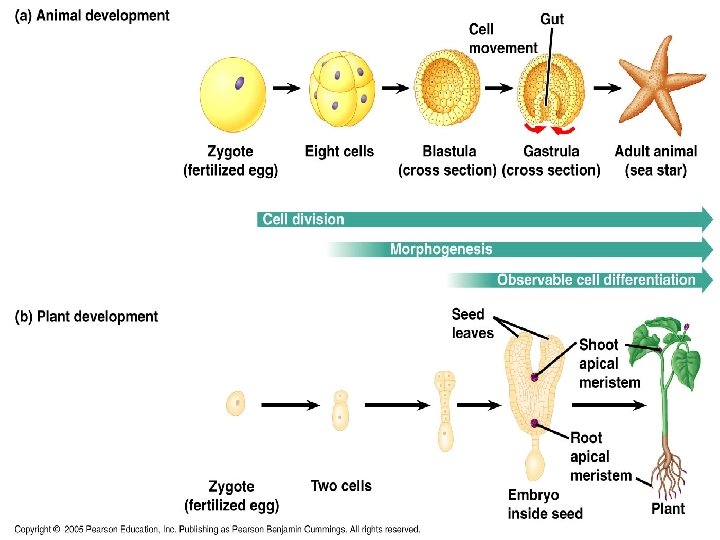
Cell Differentiation The process by which cells become specialized in form and function. These cells undergo changes that organize them into tissues and organs. Morphogenesis As the dividing cells begin to take form, they are undergoing morphogenesis which means the “creation of form. ” Morphogenetic events lay out the development very early on in development as cell division, cell differentiation and morphogenesis overlap

Morphogenesis • These morphogenetic events “tell” the organism where the head and tail are, which is the front and back, and what is left and right. • As time progresses, later morphogenetic events will give instructions as to where certain appendages will be located.

Morphogenetic Events • Morphogenetic events, as well as cell division and differentiation, take place in all multicellular organisms. • In plants, morphogenesis and growth in overall size are not limited to embryonic and juvenile periods, they occur throughout the life of the plant. • For example, apical meristems of plants are responsible for a plant’s continued growth and development and the formation of new organs throughout the plant’s life. These are perpetually embryonic regions in the tips of shoots and roots.



Cloning • Using the somatic cells of a multicellular organism to generate a new organism is • Each clone is genetically identical to the parent plant.

Microcutting propagation The production of shoots from pre-existing meristems only.

Organogenesis • The ability of nonmeristematic plant tissues to form various organs de novo. • The formation of adventitious organs • The production of roots, shoots or leaves • These organs may arise out of pre-existing meristems or out of differentiated cells • This may involve a callus intermediate but often occurs without callus.


Indirect organogenesis Explant Callus Meristemoid Primordium

Direct Organogenesis Direct shoot/root formation from the explant

Somatic Embryogenesis • The formation of adventitious embryos • The production of embryos from somatic or “non-germ” cells. • It usually involves a callus intermediate stage which can result in variation among seedlings

Types of embryogenic cells • Pre-embryogenic determined cells, PEDCs – The cells are committed to embryonic development and need only to be released. Such cells are found in embryonic tissue. • Induced embryogenic determined cells, IEDCs – In majority of cases embryogenesis is through indirect method. – Specific growth regulator concentrations and/or cultural conditions are required for initiation of callus and then redetermination of these cells into the embryogenic pattern of development.

Various terms for non-zygotic embryos ü Adventious embryos Somatic embryos arising directly from other organs or embryos. ü Parthenogenetic embryos (apomixis) Somatic embryos are formed by the unfertilized egg. ü Androgenetic embryos Somatic embryos are formed by the male gametophyte.

Somatic Embryogenesis and Organogenesis • Both of these technologies can be used as methods of micropropagation. • It is not always desirable because they may not always result in populations of identical plants. • The most beneficial use of somatic embryogenesis and organogenesis is in the production of whole plants from a single cell (or a few cells).

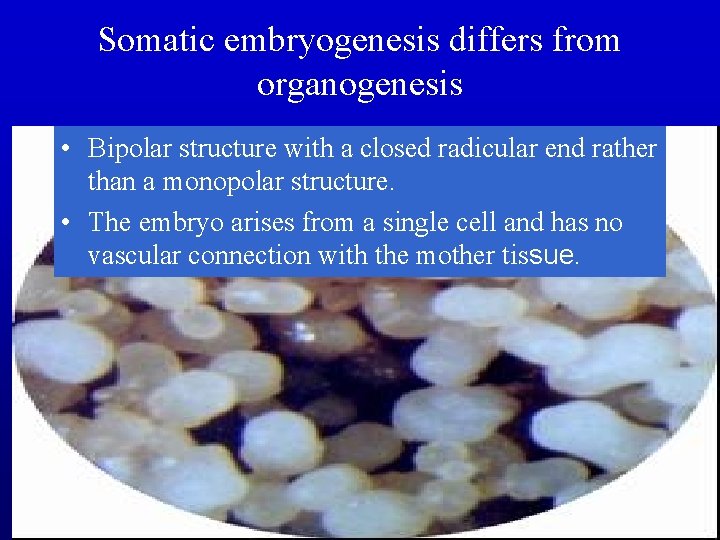
Somatic embryogenesis differs from organogenesis • Bipolar structure with a closed radicular end rather than a monopolar structure. • The embryo arises from a single cell and has no vascular connection with the mother tissue.

Two routes to somatic embryogenesis (Sharp et al. , 1980) • Direct embryogenesis – Embryos initiate directly from explant in the absence of callus formation. • Indirect embryogenesis – Callus from explant takes place from which embryos are developed.

Direct somatic embryogenesis Direct embryo formation from an explant

Indirect Somatic Embryogenesis Explant → Callus Embryogenic → Maturation → Germination 1. Calus induction 2. Callus embryogenic development 3. Multiplication 4. Maturation 5. Germination

Induction • Auxins required for induction – Proembryogenic masses form – 2, 4 -D most used – NAA, dicamba also used

Development ü Auxin must be removed for embryo development ü Continued use of auxin inhibits embryogenesis ü Stages are similar to those of zygotic embryogenesis – – – Globular Heart Torpedo Cotyledonary Germination (conversion)

Maturation • Require complete maturation with apical meristem, radicle, and cotyledons • Often obtain repetitive embryony • Storage protein production necessary • Often require ABA for complete maturation • ABA often required for normal embryo morphology – Fasciation – Precocious germination

Germination • May only obtain 3 -5% germination • Sucrose (10%), mannitol (4%) may be required • Drying (desiccation) – ABA levels decrease – Woody plants – Final moisture content 10 -40% • Chilling – Decreases ABA levels – Woody plants

Somatic embryogenesis as a means of propagation is seldom used ü High probability of mutations ü The method is usually rather difficult. ü Losing regenerative capacity become greater with repeated subculture ü Induction of embryogenesis is very difficult with many plant species. ü A deep dormancy often occurs with somatic embryogenesis

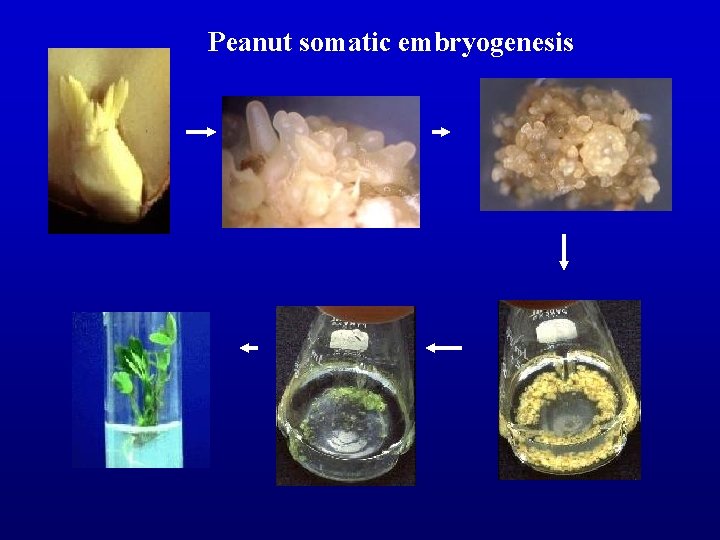
Peanut somatic embryogenesis

Steps of Micropropagation • Stage 0 – Selection & preparation of the mother plant – sterilization of the plant tissue takes place • Stage I - Initiation of culture – explant placed into growth media • Stage II - Multiplication – explant transferred to shoot media; shoots can be constantly divided • Stage III - Rooting – explant transferred to root media • Stage IV - Transfer to soil – explant returned to soil; hardened off
 Whole school whole community whole child model
Whole school whole community whole child model Non sterile compounding examples
Non sterile compounding examples Whole part whole practice
Whole part whole practice Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Objective of plant breeding
Objective of plant breeding Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Taichum
Taichum Albugo eye
Albugo eye Plant introduction in plant breeding
Plant introduction in plant breeding Are mules sterile
Are mules sterile What is this
What is this Principles of sterile technique
Principles of sterile technique Sterile supply workflow
Sterile supply workflow Chapter 15:7 cleaning with an ultrasonic unit
Chapter 15:7 cleaning with an ultrasonic unit What part of the surgical gown is considered sterile
What part of the surgical gown is considered sterile Are ligers sterile
Are ligers sterile Sterile processing
Sterile processing Sterile technique quiz
Sterile technique quiz Sterile gloves
Sterile gloves Aorn standards for surgical skin prep
Aorn standards for surgical skin prep Geometric dilution method
Geometric dilution method What is sterility
What is sterility Changement aiguille de hubert
Changement aiguille de hubert Sterile pyuria in tb
Sterile pyuria in tb Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Large volume parentrals
Large volume parentrals Chapter 15:5 sterilizing with an autoclave
Chapter 15:5 sterilizing with an autoclave Filtrazione sterile
Filtrazione sterile Sterile semisolid preparations for ophthalmic use only are
Sterile semisolid preparations for ophthalmic use only are Sterile compounding calculations
Sterile compounding calculations Handout 14-1 thermometer worksheet answers
Handout 14-1 thermometer worksheet answers Opening sterile supplies
Opening sterile supplies Chapter 15:5 sterilizing with an autoclave
Chapter 15:5 sterilizing with an autoclave Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile field rules
Sterile field rules Who gmp for sterile pharmaceutical products
Who gmp for sterile pharmaceutical products Chapter 14:8 using sterile techniques
Chapter 14:8 using sterile techniques Sterile flower
Sterile flower Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile pyuria ppt
Sterile pyuria ppt Aseptic technique pharmacy
Aseptic technique pharmacy Sterile processing decontamination ppe
Sterile processing decontamination ppe 15:8 using sterile techniques
15:8 using sterile techniques Bowmans capsule
Bowmans capsule Sterile technique cell culture
Sterile technique cell culture Podning af lupiner
Podning af lupiner Low and medium risk sterile compounding quiz
Low and medium risk sterile compounding quiz All the tiny pieces
All the tiny pieces What do the six different chess pieces represent
What do the six different chess pieces represent Structural pieces
Structural pieces Setting up chess board
Setting up chess board Gcf and lcm word problems key words
Gcf and lcm word problems key words Pangaea map puzzle
Pangaea map puzzle Two pieces of advice to avoid computer addiction
Two pieces of advice to avoid computer addiction It takes ten identical pieces to form a circular track
It takes ten identical pieces to form a circular track Reusable pieces of content or document parts
Reusable pieces of content or document parts Persuasive non fiction
Persuasive non fiction 3 pieces of paper
3 pieces of paper To stir ingredients until they are thoroughly combined
To stir ingredients until they are thoroughly combined It is a self portrait composed of many pieces
It is a self portrait composed of many pieces Reese's pieces simulation
Reese's pieces simulation Process of joining two metals
Process of joining two metals Putting all the pieces together
Putting all the pieces together Pattern guide sheet
Pattern guide sheet In maths
In maths Analisis pieces
Analisis pieces Operating system three easy pieces
Operating system three easy pieces