Experiments to observe diffusion in action 1 Diffusion

Experiments to observe diffusion in action 1. Diffusion across the lining of the small intestine. 2. Diffusion into and out of cells Aim This experiment demonstrates how dissolved food particles move across the lining of the small intestine and into the blood. © Boardworks Ltd 2003
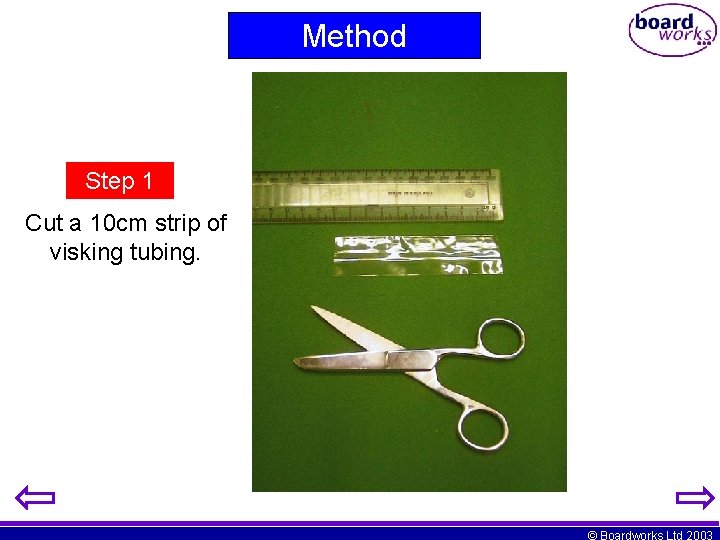
Method Step 1 Cut a 10 cm strip of visking tubing. © Boardworks Ltd 2003

Method Step 2 Wet it with water and rub it between your fingers until it opens at both ends. Hold one of the ends close to your mouth and blow through the tube so that it opens from one end to the other. © Boardworks Ltd 2003

Method Step 3 Cut a small piece of cotton and tie one end of the visking tubing tightly. It is essential that this tie is watertight. To check, pour some water into the tube and check for any holes © Boardworks Ltd 2003

Method Step 4 Measure 2 cm 3 of starch solution into a glass beaker (to confirm this, perform the starch test on a small sample of liquid - see step 13). This will be your food (carbohydrate) sample. © Boardworks Ltd 2003

Method Step 5 Measure out 2 cm 3 of amylase solution. This will be your sample of carbohydrase enzyme. Step 6 The next steps will require considerable skill because the equipment is rather tricky to handle. Carefully pour the enzyme solution into the beaker containing the food solution. Then give this new solution a gentle stir. Cut a new piece of cotton. © Boardworks Ltd 2003

Method Step 7 Pour the solution into the visking tubing. Pinch the open end to stop the solution leaking out of the tube. © Boardworks Ltd 2003

Method Step 8 Now use the cotton to tie-up the open end of the visking tubing. This will complete the preparation of the model of the small intestine. We have a tube filled with food and enzymes in solution. © Boardworks Ltd 2003

Method Step 9 Now place the sealed visking tubing into a glass boiling tube. Ensure that the tube is placed in far enough so that it sits below the opening of the boiling tube. © Boardworks Ltd 2003

Method Step 10 Next, add enough water to the boiling tube to ensure that the visking tube is completely submerged. This water will represent the blood that flows around the outer lining of the small intestine. © Boardworks Ltd 2003

Method Step 11 The sealed tube is now left, submerged in the water for 10 minutes. This will provide enough time for the enzymes within the bag to digest the food. In this case the starch will be broken down into sugars. The concentration of sugar inside the bag will then increase. As the bag is surrounded by water, a concentration gradient will be produced. The diagram will explain the process. © Boardworks Ltd 2003
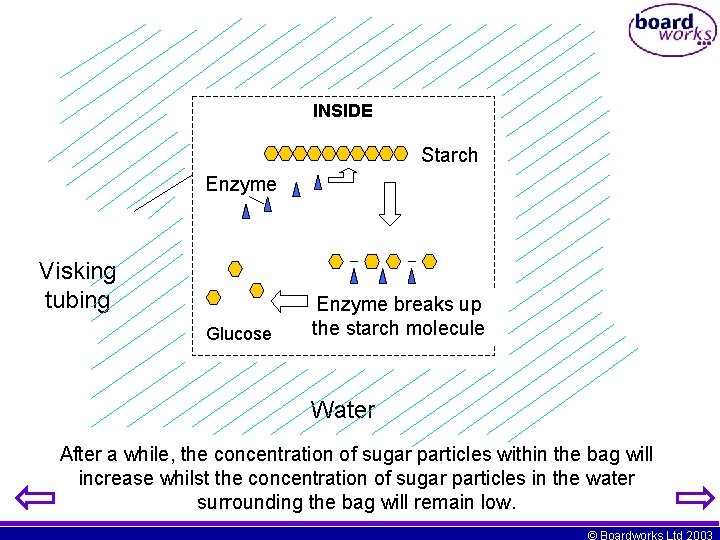
INSIDE Starch Enzyme Visking tubing Glucose Enzyme breaks up the starch molecule Water After a while, the concentration of sugar particles within the bag will increase whilst the concentration of sugar particles in the water surrounding the bag will remain low. © Boardworks Ltd 2003
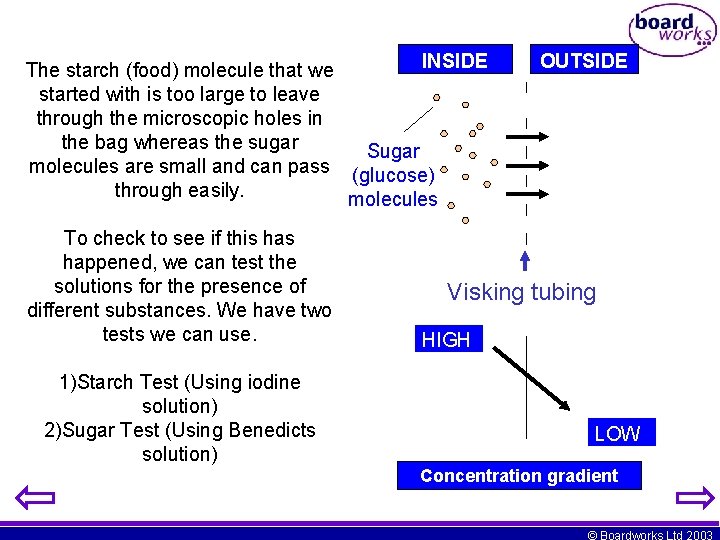
INSIDE The starch (food) molecule that we started with is too large to leave through the microscopic holes in the bag whereas the sugar Sugar molecules are small and can pass (glucose) through easily. molecules To check to see if this happened, we can test the solutions for the presence of different substances. We have two tests we can use. 1)Starch Test (Using iodine solution) 2)Sugar Test (Using Benedicts solution) OUTSIDE Visking tubing HIGH LOW Concentration gradient © Boardworks Ltd 2003
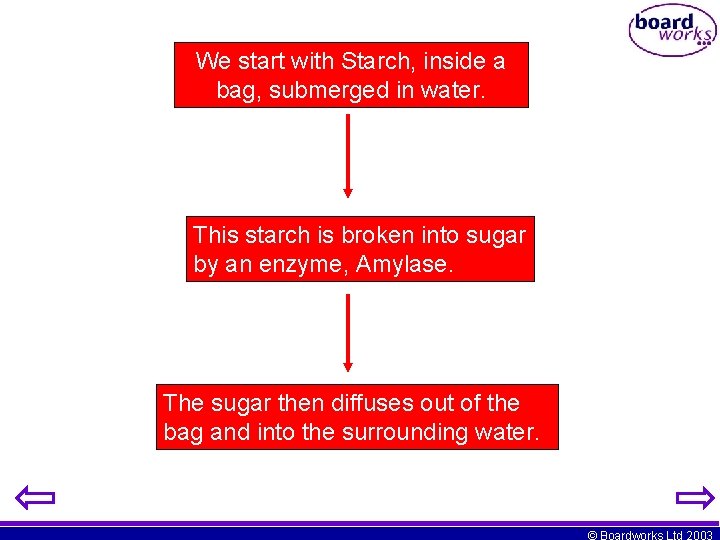
We start with Starch, inside a bag, submerged in water. This starch is broken into sugar by an enzyme, Amylase. The sugar then diffuses out of the bag and into the surrounding water. © Boardworks Ltd 2003
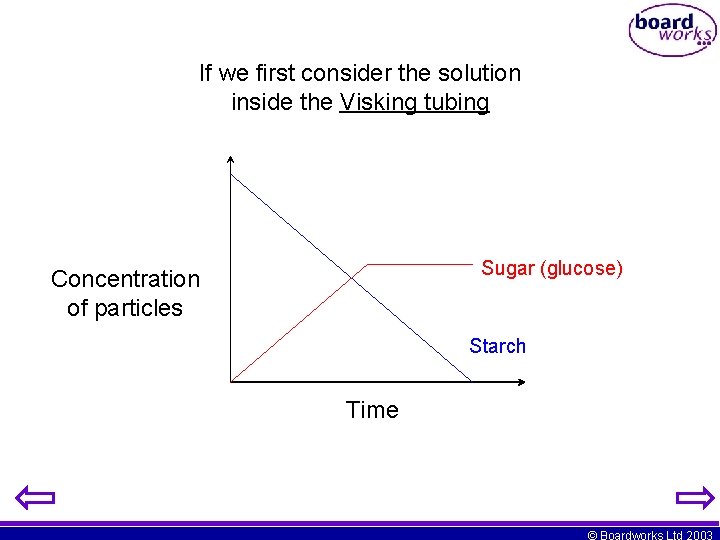
If we first consider the solution inside the Visking tubing Sugar (glucose) Concentration of particles Starch Time © Boardworks Ltd 2003
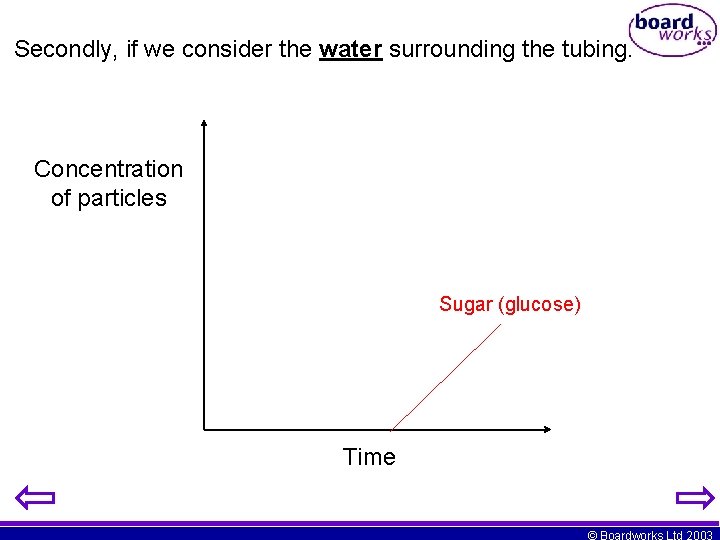
Secondly, if we consider the water surrounding the tubing. Concentration of particles Sugar (glucose) Time © Boardworks Ltd 2003

Step 12 Method Remove the sealed tubing from the boiling tube, taking care not to spill the surrounding water. Open the tube and pour the contents into a fresh glass beaker. Pour the surrounding water into another glass beaker. © Boardworks Ltd 2003
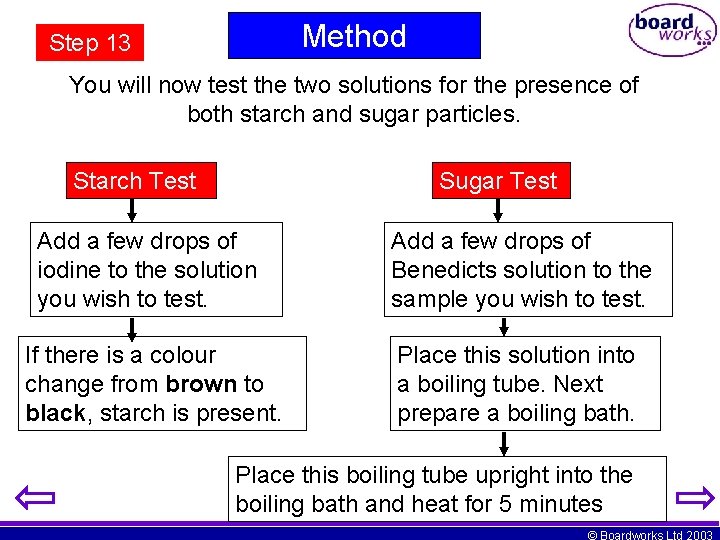
Method Step 13 You will now test the two solutions for the presence of both starch and sugar particles. Starch Test Sugar Test Add a few drops of iodine to the solution you wish to test. Add a few drops of Benedicts solution to the sample you wish to test. If there is a colour change from brown to black, starch is present. Place this solution into a boiling tube. Next prepare a boiling bath. Place this boiling tube upright into the boiling bath and heat for 5 minutes © Boardworks Ltd 2003
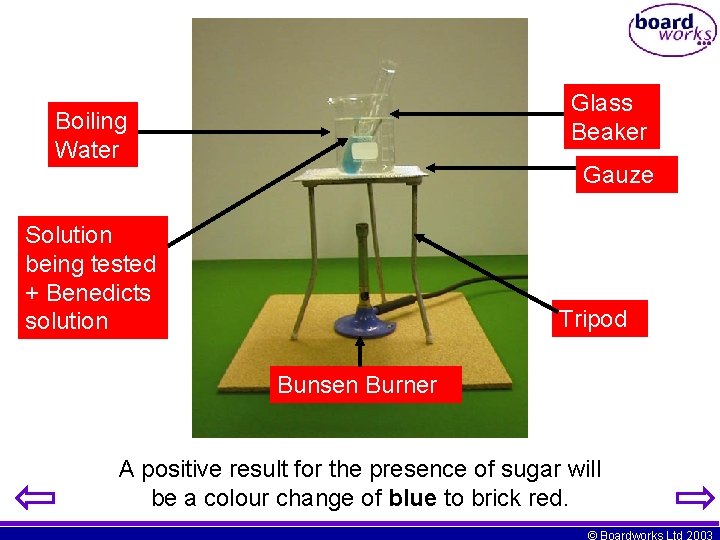
Glass Beaker Boiling Water Gauze Solution being tested + Benedicts solution Tripod Bunsen Burner A positive result for the presence of sugar will be a colour change of blue to brick red. © Boardworks Ltd 2003
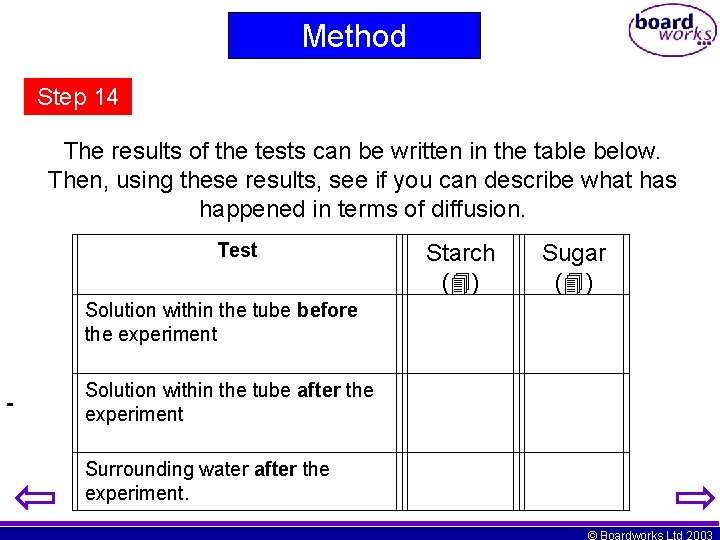
Method Step 14 The results of the tests can be written in the table below. Then, using these results, see if you can describe what has happened in terms of diffusion. Test Starch ( ) Sugar ( ) Solution within the tube before the experiment Solution within the tube after the experiment Surrounding water after the experiment. © Boardworks Ltd 2003
Experiment 2 Aim This experiment will demonstrate how living cells rely on diffusion. In this case the diffusing particle is water and as you will remember, the process is known as osmosis. We will see how plant cells depend on osmosis (diffusion of water) for obtaining vital water particles. As you will recall, the plant requires water both for photosynthesis and support. We are using potatoes as our representative plant. © Boardworks Ltd 2003
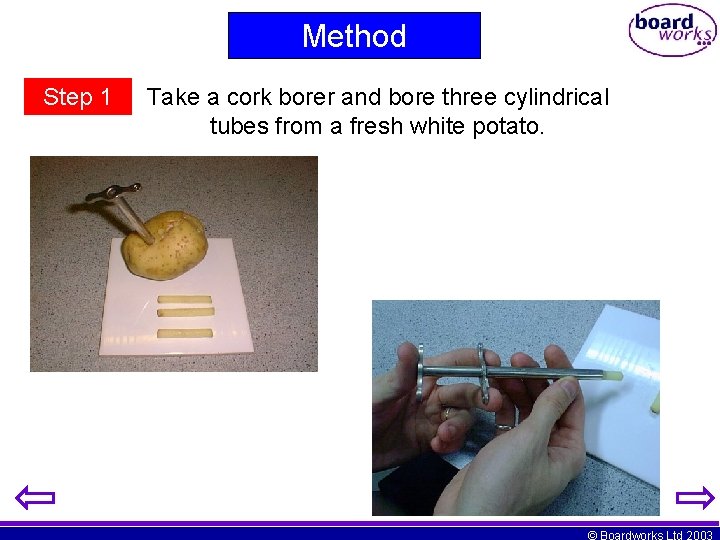
Method Step 1 Take a cork borer and bore three cylindrical tubes from a fresh white potato. © Boardworks Ltd 2003
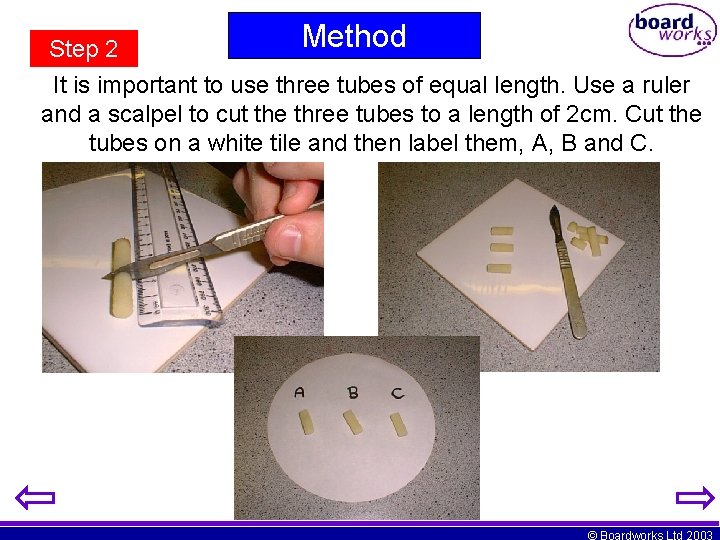
Method Step 2 It is important to use three tubes of equal length. Use a ruler and a scalpel to cut the three tubes to a length of 2 cm. Cut the tubes on a white tile and then label them, A, B and C. © Boardworks Ltd 2003
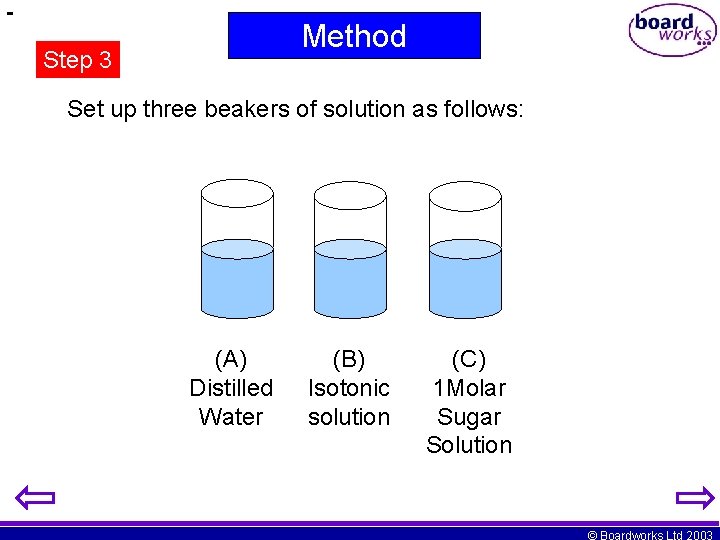
Method Step 3 Set up three beakers of solution as follows: (A) Distilled Water (B) Isotonic solution (C) 1 Molar Sugar Solution © Boardworks Ltd 2003
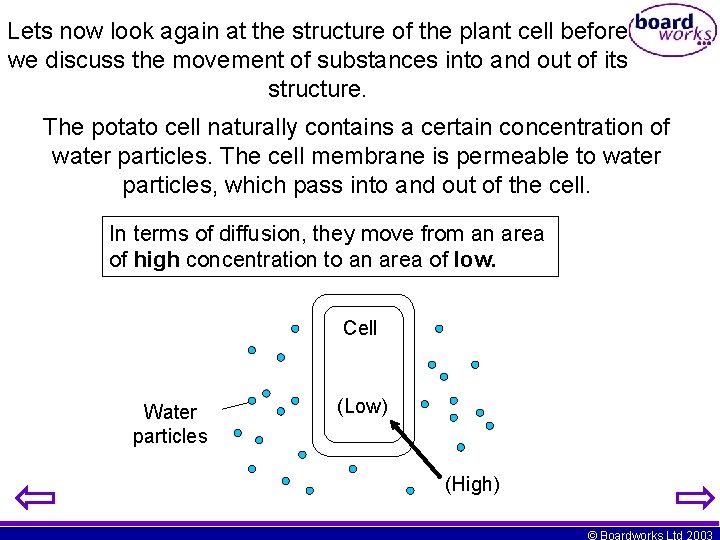
Lets now look again at the structure of the plant cell before we discuss the movement of substances into and out of its structure. The potato cell naturally contains a certain concentration of water particles. The cell membrane is permeable to water particles, which pass into and out of the cell. In terms of diffusion, they move from an area of high concentration to an area of low. Cell Water particles (Low) (High) © Boardworks Ltd 2003
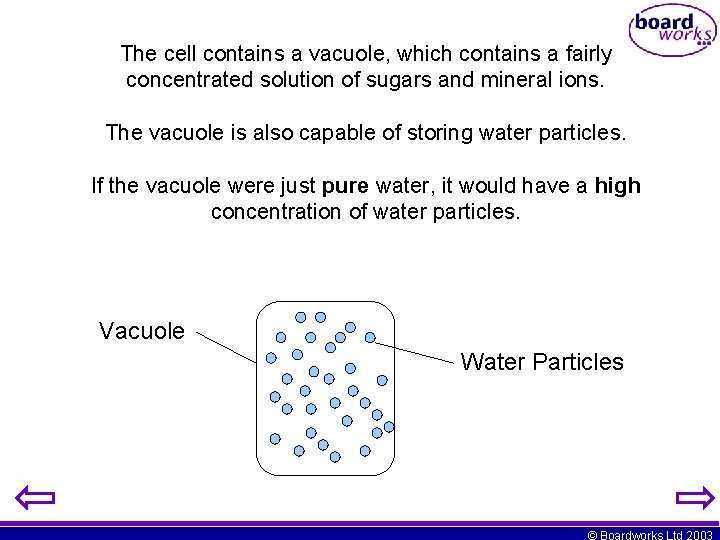
The cell contains a vacuole, which contains a fairly concentrated solution of sugars and mineral ions. The vacuole is also capable of storing water particles. If the vacuole were just pure water, it would have a high concentration of water particles. Vacuole Water Particles © Boardworks Ltd 2003
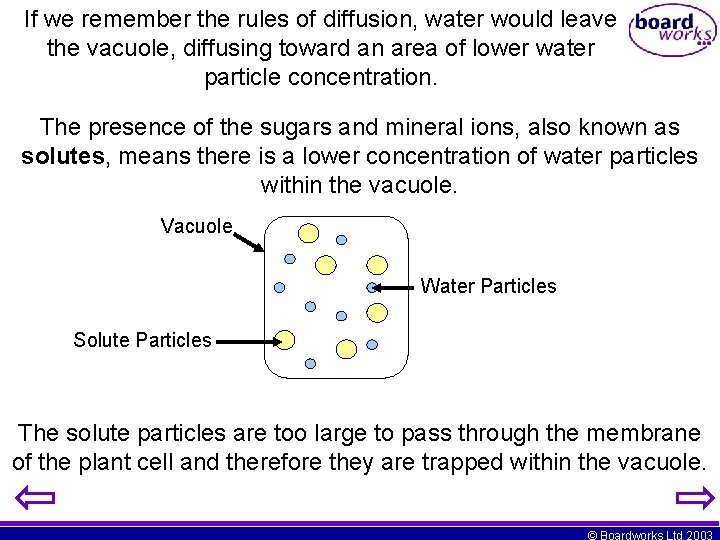
If we remember the rules of diffusion, water would leave the vacuole, diffusing toward an area of lower water particle concentration. The presence of the sugars and mineral ions, also known as solutes, means there is a lower concentration of water particles within the vacuole. Vacuole Water Particles Solute Particles The solute particles are too large to pass through the membrane of the plant cell and therefore they are trapped within the vacuole. © Boardworks Ltd 2003
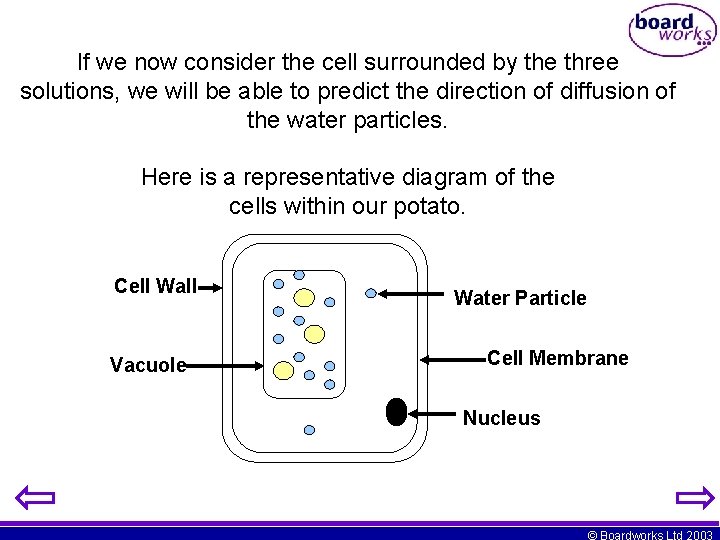
If we now consider the cell surrounded by the three solutions, we will be able to predict the direction of diffusion of the water particles. Here is a representative diagram of the cells within our potato. Cell Wall Vacuole Water Particle Cell Membrane Nucleus © Boardworks Ltd 2003
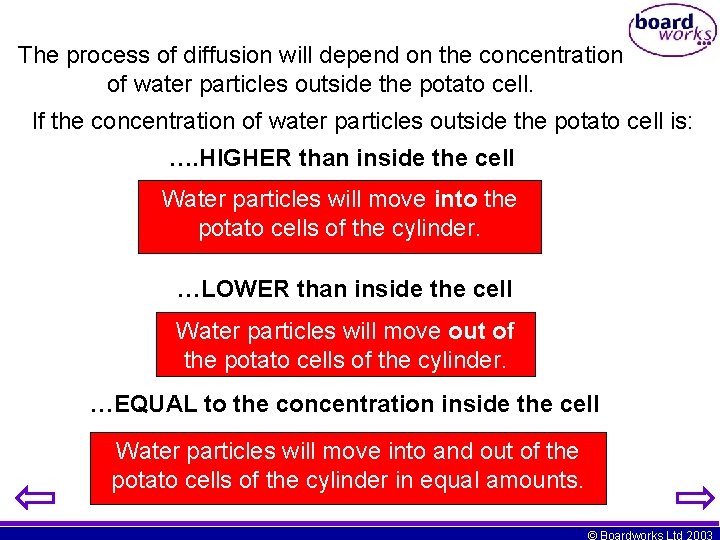
The process of diffusion will depend on the concentration of water particles outside the potato cell. If the concentration of water particles outside the potato cell is: …. HIGHER than inside the cell Water particles will move into the potato cells of the cylinder. …LOWER than inside the cell Water particles will move out of the potato cells of the cylinder. …EQUAL to the concentration inside the cell Water particles will move into and out of the potato cells of the cylinder in equal amounts. © Boardworks Ltd 2003
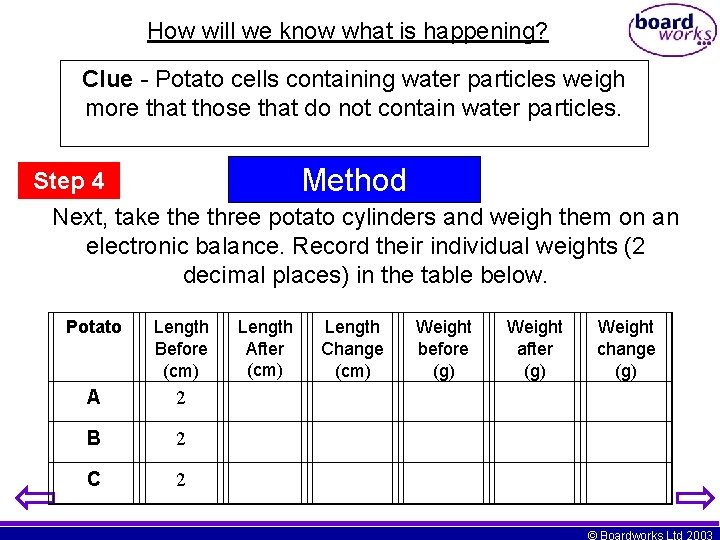
How will we know what is happening? Clue - Potato cells containing water particles weigh more that those that do not contain water particles. Step 4 Method Next, take three potato cylinders and weigh them on an electronic balance. Record their individual weights (2 decimal places) in the table below. Potato Length Before (cm) A 2 B 2 C 2 Length After (cm) Length Change (cm) Weight before (g) Weight after (g) Weight change (g) © Boardworks Ltd 2003

Step 5 Method Once the 3 potatoes have been measured and weighed, they are to be placed into their respective solutions, A going into solution A (distilled water) and so on. Remember that the isotonic solution is so called because it contains an identical concentration of particles to that of the potato cell. As soon as the potato cylinders are placed into the solutions, you must begin timing. The potato cylinders are to be left submerged in the solutions for 10 minutes. © Boardworks Ltd 2003

Step 6 Method When the potatoes have been in the solutions for 10 minutes, carefully remove each one with a pair of tweezers, making sure that you remember which potato was in which solution. © Boardworks Ltd 2003

Step 7 Method Shake off the excess water that is covering the outside of the potato. This is will affect the results and is not related to the diffusion process. Use a piece of blotting paper to absorb the drips. Step 8 Place the cylinders onto a white tile and carefully measure the length of each piece. Record the results in the results table. © Boardworks Ltd 2003

Step 9 Method Finally, re-weigh the potato cylinders and record the weight change (if any) in the results table. Once you have gathered all the results, you can now evaluate the results in terms of the diffusion of water particles into and out of the potato cells. © Boardworks Ltd 2003
- Slides: 34