EXPERIMENTAL YEILD IN THE REACTION Na 2 CO

- Slides: 6

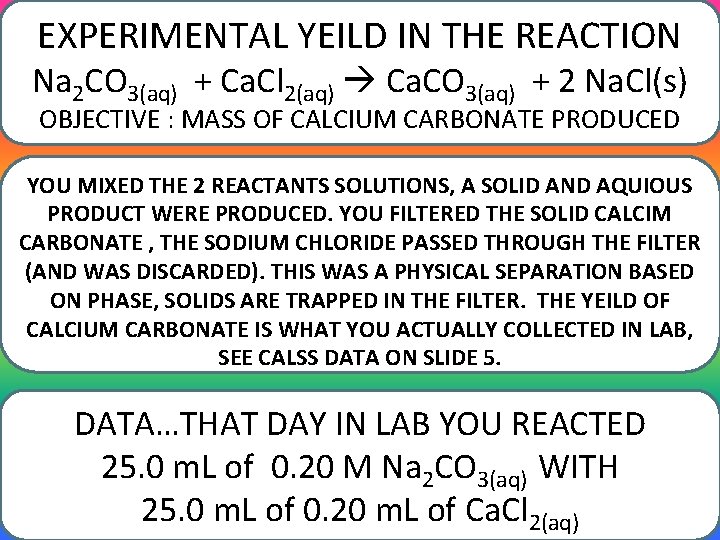
EXPERIMENTAL YEILD IN THE REACTION Na 2 CO 3(aq) + Ca. Cl 2(aq) Ca. CO 3(aq) + 2 Na. Cl(s) OBJECTIVE : MASS OF CALCIUM CARBONATE PRODUCED YOU MIXED THE 2 REACTANTS SOLUTIONS, A SOLID AND AQUIOUS PRODUCT WERE PRODUCED. YOU FILTERED THE SOLID CALCIM CARBONATE , THE SODIUM CHLORIDE PASSED THROUGH THE FILTER (AND WAS DISCARDED). THIS WAS A PHYSICAL SEPARATION BASED ON PHASE, SOLIDS ARE TRAPPED IN THE FILTER. THE YEILD OF CALCIUM CARBONATE IS WHAT YOU ACTUALLY COLLECTED IN LAB, SEE CALSS DATA ON SLIDE 5. DATA…THAT DAY IN LAB YOU REACTED 25. 0 m. L of 0. 20 M Na 2 CO 3(aq) WITH 25. 0 m. L of 0. 20 m. L of Ca. Cl 2(aq)

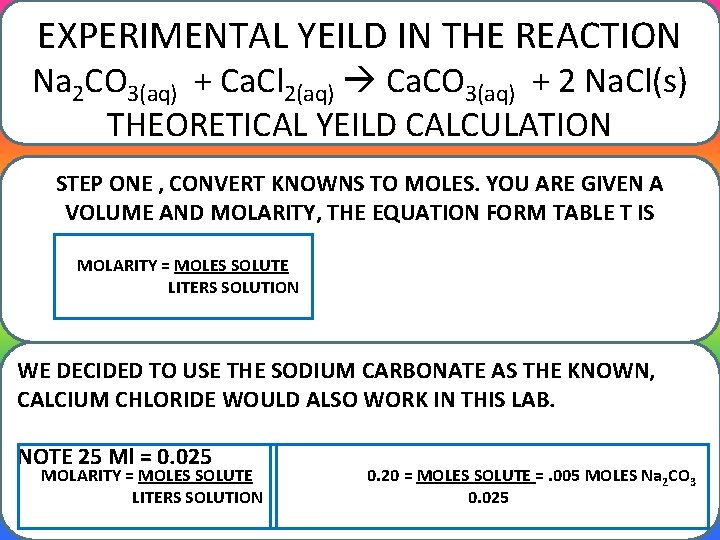
EXPERIMENTAL YEILD IN THE REACTION Na 2 CO 3(aq) + Ca. Cl 2(aq) Ca. CO 3(aq) + 2 Na. Cl(s) THEORETICAL YEILD CALCULATION STEP ONE , CONVERT KNOWNS TO MOLES. YOU ARE GIVEN A VOLUME AND MOLARITY, THE EQUATION FORM TABLE T IS MOLARITY = MOLES SOLUTE LITERS SOLUTION WE DECIDED TO USE THE SODIUM CARBONATE AS THE KNOWN, CALCIUM CHLORIDE WOULD ALSO WORK IN THIS LAB. NOTE 25 Ml = 0. 025 MOLARITY = MOLES SOLUTE LITERS SOLUTION 0. 20 = MOLES SOLUTE =. 005 MOLES Na 2 CO 3 0. 025

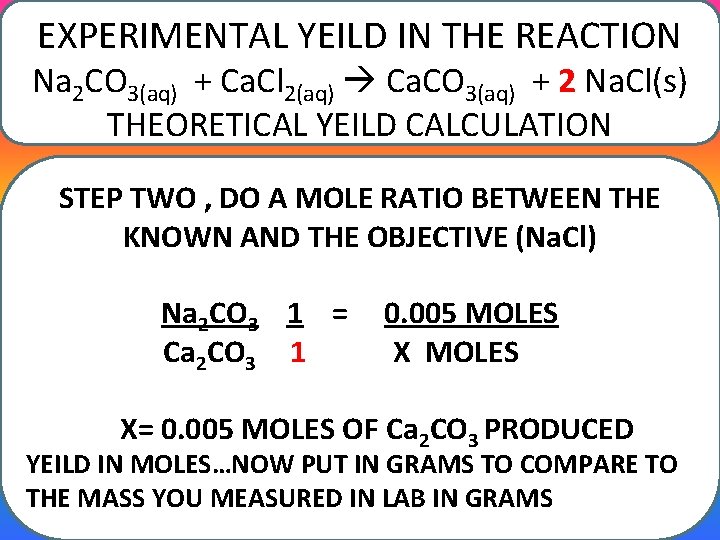
EXPERIMENTAL YEILD IN THE REACTION Na 2 CO 3(aq) + Ca. Cl 2(aq) Ca. CO 3(aq) + 2 Na. Cl(s) THEORETICAL YEILD CALCULATION STEP TWO , DO A MOLE RATIO BETWEEN THE KNOWN AND THE OBJECTIVE (Na. Cl) Na 2 CO 3 1 = Ca 2 CO 3 1 0. 005 MOLES X= 0. 005 MOLES OF Ca 2 CO 3 PRODUCED YEILD IN MOLES…NOW PUT IN GRAMS TO COMPARE TO THE MASS YOU MEASURED IN LAB IN GRAMS

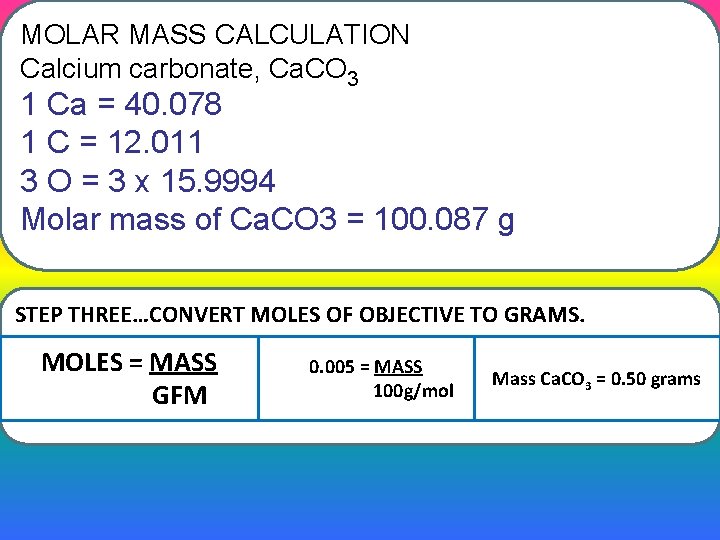
MOLAR MASS CALCULATION Calcium carbonate, Ca. CO 3 1 Ca = 40. 078 1 C = 12. 011 3 O = 3 x 15. 9994 Molar mass of Ca. CO 3 = 100. 087 g STEP THREE…CONVERT MOLES OF OBJECTIVE TO GRAMS. MOLES = MASS GFM 0. 005 = MASS 100 g/mol Mass Ca. CO 3 = 0. 50 grams

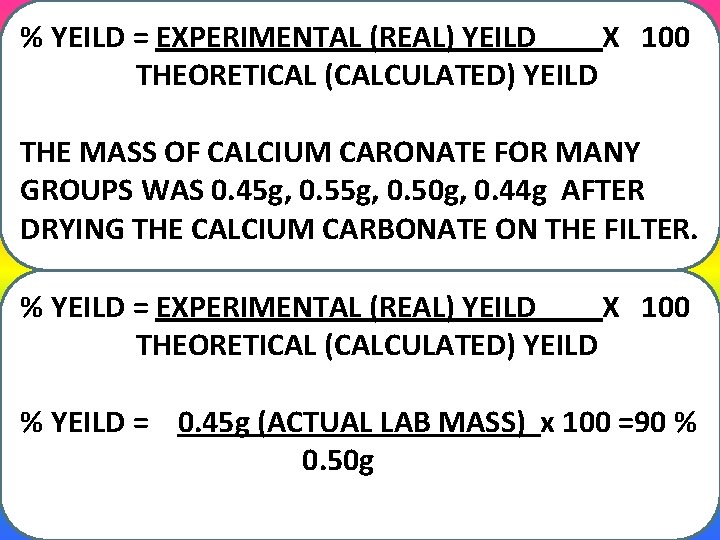
% YEILD = EXPERIMENTAL (REAL) YEILD X 100 THEORETICAL (CALCULATED) YEILD THE MASS OF CALCIUM CARONATE FOR MANY GROUPS WAS 0. 45 g, 0. 50 g, 0. 44 g AFTER DRYING THE CALCIUM CARBONATE ON THE FILTER. % YEILD = EXPERIMENTAL (REAL) YEILD X 100 THEORETICAL (CALCULATED) YEILD % YEILD = 0. 45 g (ACTUAL LAB MASS) x 100 =90 % 0. 50 g

% YEILD = EXPERIMENTAL (REAL) YEILD x THEORETICAL 100 THEORETICAL (CALCULATED) YEILD X YOUSE YOUR DATA OR THE CLASS DATA TO CALCULATE YOUR YEILD…YOUR LAB MUST INCLUDE EXTENSIVE DISCUSSION OF THE CALCULATION.