Experimental Study Experimental epidemiology Prospective study The randomly














- Slides: 22

Experimental Study

Experimental epidemiology • Prospective study • The randomly assigned subjects to either treatment or certain interventions are under the direct control of the investigator • The controls are assigned for comparison and assessing the effectiveness of interventions • It involves some action, intervention or manipulation

Aims • To provide "scientific proof" of aetiological factors which may permit the modification or control of the disease (s). • To provide a method of measuring the effectiveness and efficiency of health services/programs for the prevention, control and treatment of disease and improve the health of the community. • To study the efficacy and effectiveness of drugs/vaccines for the treatment and prevention of disease or health problems.

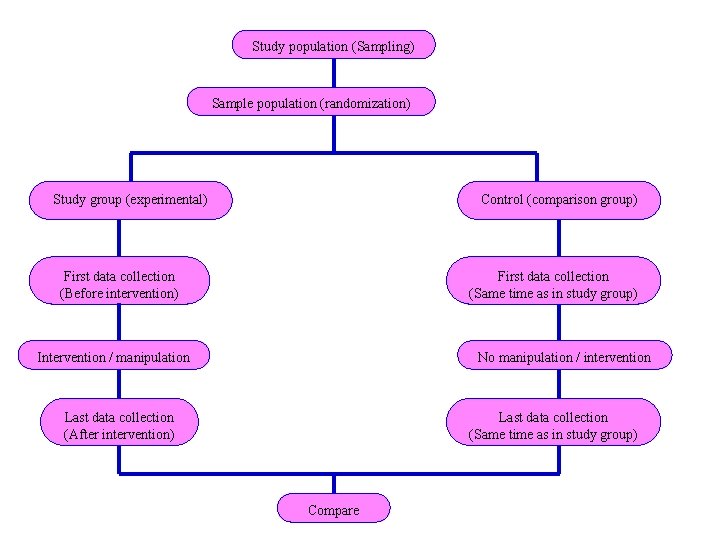
Study population (Sampling) Sample population (randomization) Study group (experimental) Control (comparison group) First data collection (Before intervention) First data collection (Same time as in study group) Intervention / manipulation No manipulation / intervention Last data collection (After intervention) Last data collection (Same time as in study group) Compare

• Experimental studies are conducted either in animal or humans. • Animal studies are used in anatomy, physiology, pathology, microbiology, immunology, genetics, chemotherapy etc. • Animal studies are used for – Experimental reproduction of human disease in animal to confirm aetiological hypothesis and to study the pathogenic phenomena or mechanisms – Testing the efficacy of preventive and therapeutic measures such as drugs and vaccines – Completing the natural history of disease

• Human experiments are needed to investigate disease aetiology and to evaluate the preventive and therapeutic measure for that disease which cannot be reproduced in animal. • Experimental studies are of two types I. Randomized controlled trails II. Non-randomized controlled trails

Randomized controlled trial • Randomized controlled trail (RCT) is scientific technique to evaluate method of treatment and prevention. • Steps of RCT – Drawing up a protocol – Selecting reference and experimental populations – Randomization – Manipulation and intervention – Follow up – Assessment of outcome

Drawing up a protocol • RCT is conducted under a strict protocol • The protocol should include – aims and objectives of the study – selection criteria of study and control group – detailed procedure • The protocol should be followed throughout the study

Selection of reference and experimental populations i. Reference population – – It is the population in which finding of trials, if successful, are applied. Reference population may be board or limited specifically by geography, age, sex, occupation, social group etc. ii. Experimental population – – The study population is derived from the reference population to generalize the study findings to the target population. They must give informed consent They should be representative of the population They should be qualified for the trail

• The participants are randomly assigned into study and control group. • Study group is the group of individuals who are offered the new treatment, preventive agent or any type of intervention under investigation. • Control group is the group of individuals who are not offered the measure and receive the usual accepted treatment or placebo.

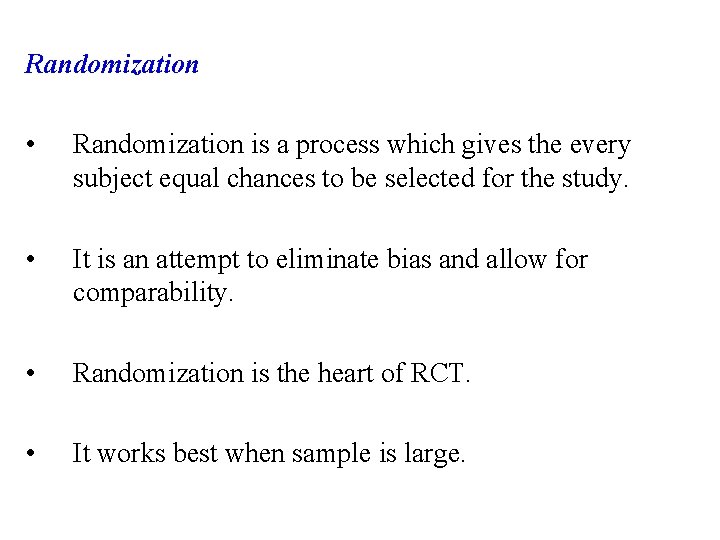
Randomization • Randomization is a process which gives the every subject equal chances to be selected for the study. • It is an attempt to eliminate bias and allow for comparability. • Randomization is the heart of RCT. • It works best when sample is large.

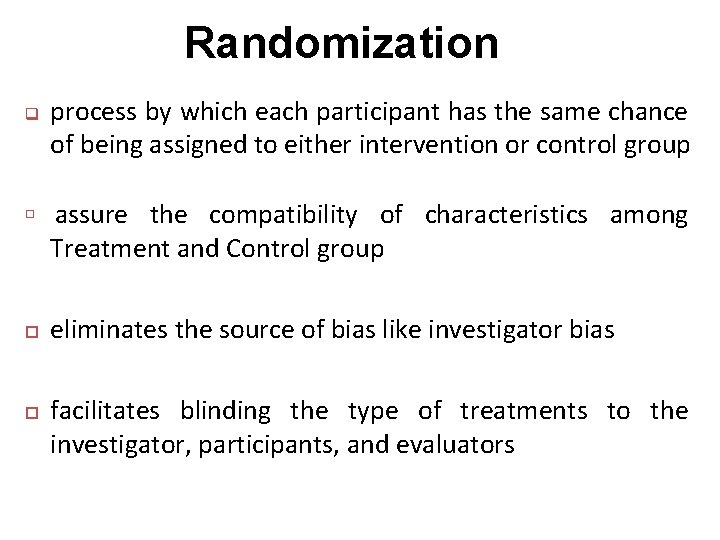
Randomization q process by which each participant has the same chance of being assigned to either intervention or control group assure the compatibility of characteristics among Treatment and Control group eliminates the source of bias like investigator bias facilitates blinding the type of treatments to the investigator, participants, and evaluators

Manipulation • It is the intervention stage, which is performed after the selection of study and control group. Follow up • It is essential till final assessment of outcome. • During follow up, there may be attrition problem due to death, migration, and loss of interest.

Assessment of outcome • The final step is assessment of the outcome in terms of positive and negative results. • Bias during assessment may be due to subject variation, observer bias and bias in evaluation. • Blinding is done to reduce bias in outcome assessment. – In single blind trial, the participant is not aware whether belongs to the study group or control group. – In double blind trial, both the doctor or study team member and participants do not know the group allocation and treatment received. – In triple blind trial, the participants, investigator and analyzer are all blind.

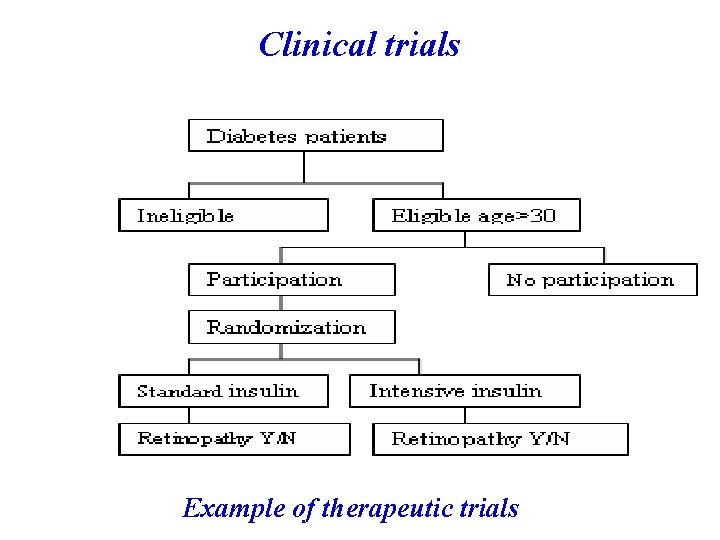
Clinical trials Example of therapeutic trials

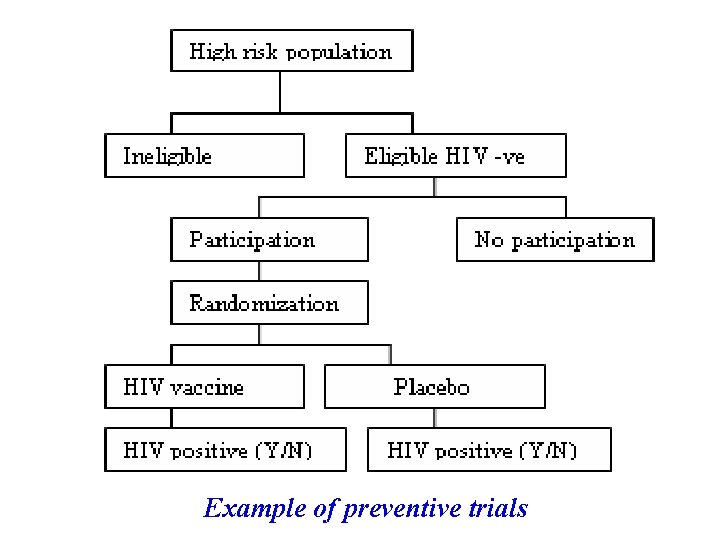
Example of preventive trials

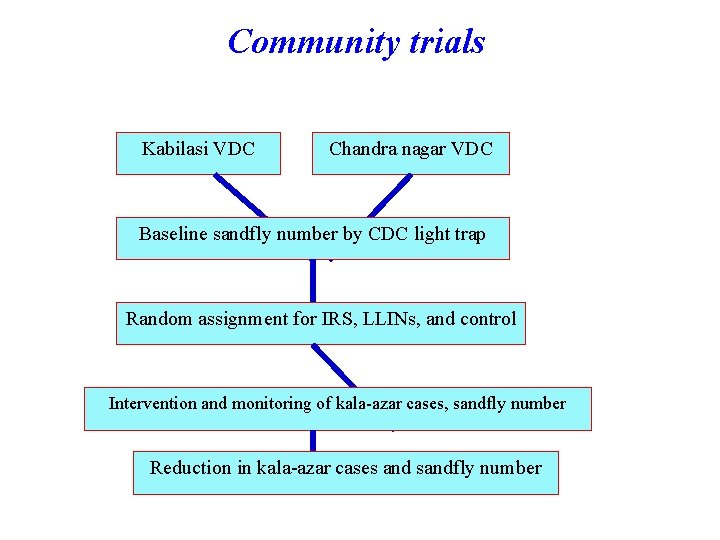
Community trials Kabilasi VDC Chandra nagar VDC Baseline sandfly number by CDC light trap Random assignment for IRS, LLINs, and control Intervention and monitoring of kala-azar cases, sandfly number Reduction in kala-azar cases and sandfly number

Blinding v procedure that prevent study participants, caregivers, or outcome assessors from knowing which intervention was received v 3 types • Single blind - participants are not aware of treatment group • Double blind - both participants and investigators unaware • Triple blind – participants, investigators and the person reviewing the data are all masked.

Advantages • Other study designs can also detect associations between an intervention and an outcome. But they cannot rule out the possibility that the association was caused by a third factor linked to both intervention and outcome. • It controls selection bias and confounding bias. • Facilitates effective blinding. • Carries strong evidences than any other studies (plausibility). • It also maintains the advantages of cohort studies.

Disadvantages • May be complex and expensive • Difficult and expensive with low incidence outcomes • May lack representativeness - volunteers may differ from population of interest • Ethical challenges of experimental research • Sometimes impossible or impractical to conduct

Ethical consideration Is it ethical to randomize? Is it ethical not to randomize? Is it ethical to use a placebo? Another important question is whether truly informed consent can be obtained? • Under what circumstances should a trial be stopped earlier than originally planned (either harmful effects or beneficial effects of the agent become apparent early) • •

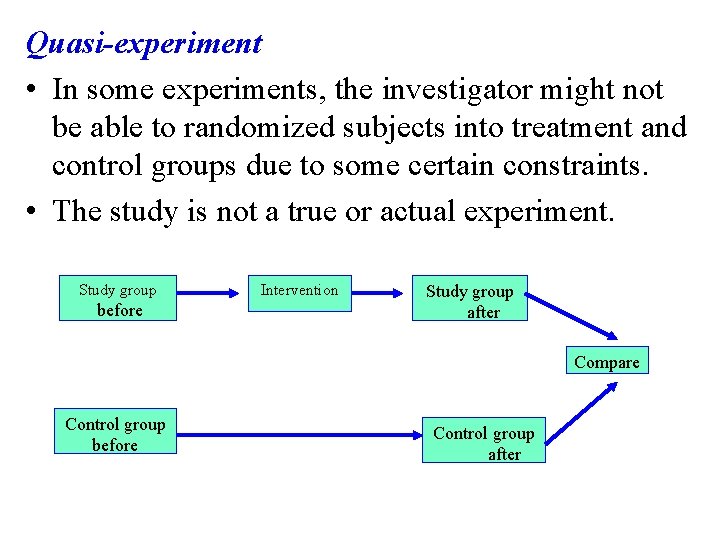
Quasi-experiment • In some experiments, the investigator might not be able to randomized subjects into treatment and control groups due to some certain constraints. • The study is not a true or actual experiment. Study group before Intervention Study group after Compare Control group before Control group after