Experimental Measurements of Collisional Cross Sections and Rates





- Slides: 16

Experimental Measurements of Collisional Cross Sections and Rates at Astrophysical and Quantum Collisional Temperatures Frank C. De Lucia Department of Physics Ohio State University Leiden Center on Herschel Preparatory Science Leiden December 5 - 7, 2006

An Experimentalist’s History and Perspective Pioneering Theory of Green and Thaddeus Explore New Experimental Regimes What is the physics in the regime where k. T ~ hnr ~Vwell?

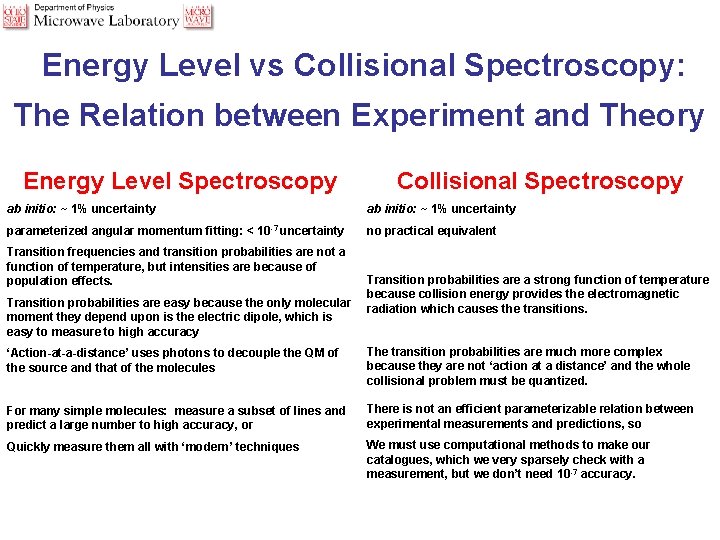
Energy Level vs Collisional Spectroscopy: The Relation between Experiment and Theory Energy Level Spectroscopy Collisional Spectroscopy ab initio: ~ 1% uncertainty parameterized angular momentum fitting: < 10 -7 uncertainty no practical equivalent Transition frequencies and transition probabilities are not a function of temperature, but intensities are because of population effects. Transition probabilities are easy because the only molecular moment they depend upon is the electric dipole, which is easy to measure to high accuracy Transition probabilities are a strong function of temperature because collision energy provides the electromagnetic radiation which causes the transitions. ‘Action-at-a-distance’ uses photons to decouple the QM of the source and that of the molecules The transition probabilities are much more complex because they are not ‘action at a distance’ and the whole collisional problem must be quantized. For many simple molecules: measure a subset of lines and predict a large number to high accuracy, or There is not an efficient parameterizable relation between experimental measurements and predictions, so Quickly measure them all with ‘modern’ techniques We must use computational methods to make our catalogues, which we very sparsely check with a measurement, but we don’t need 10 -7 accuracy.

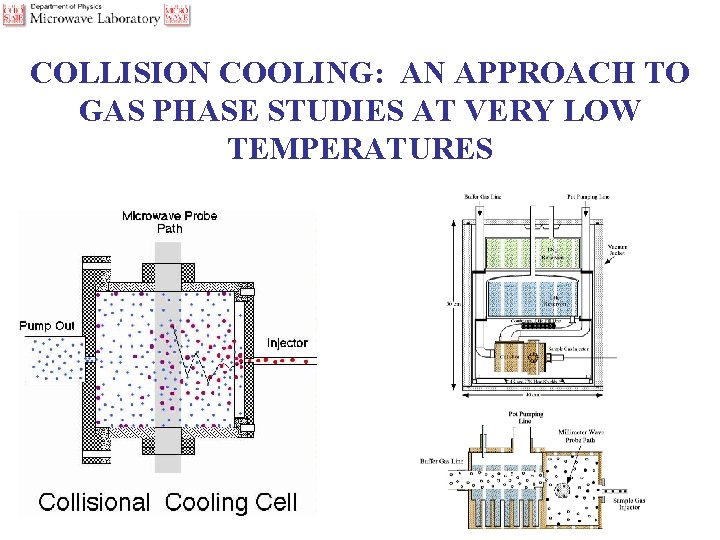
COLLISION COOLING: AN APPROACH TO GAS PHASE STUDIES AT VERY LOW TEMPERATURES

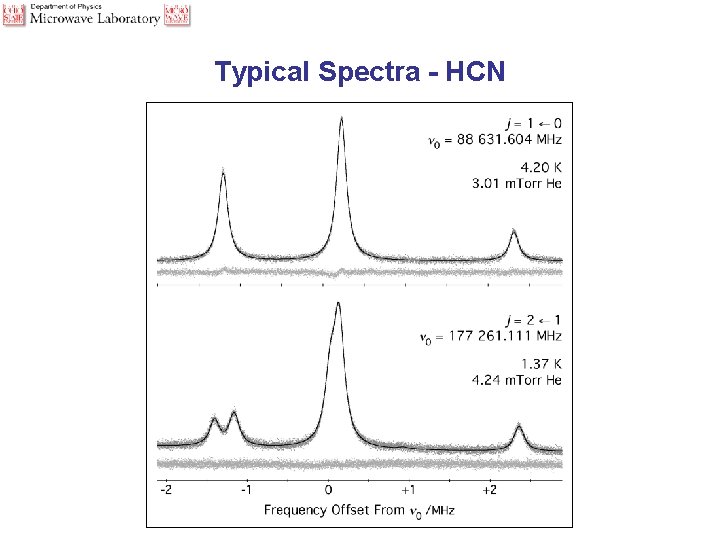
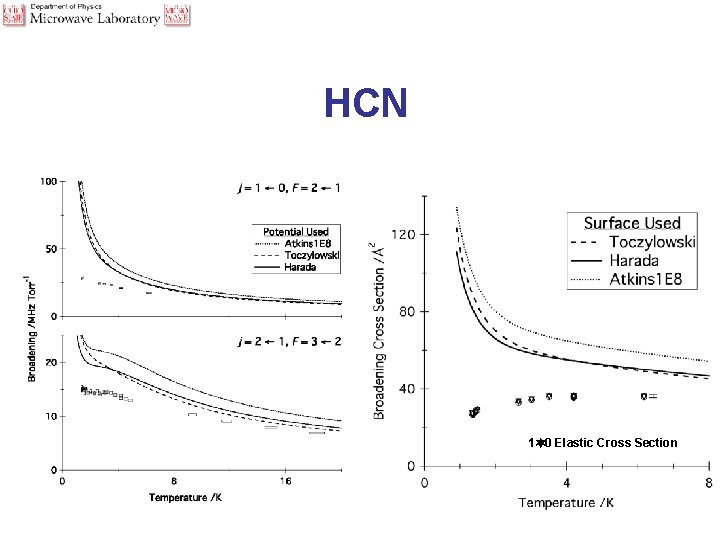
Typical Spectra - HCN

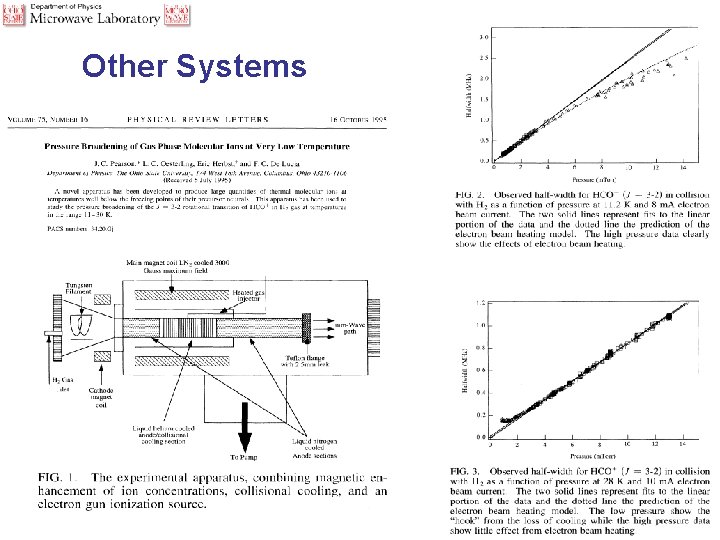
Other Systems

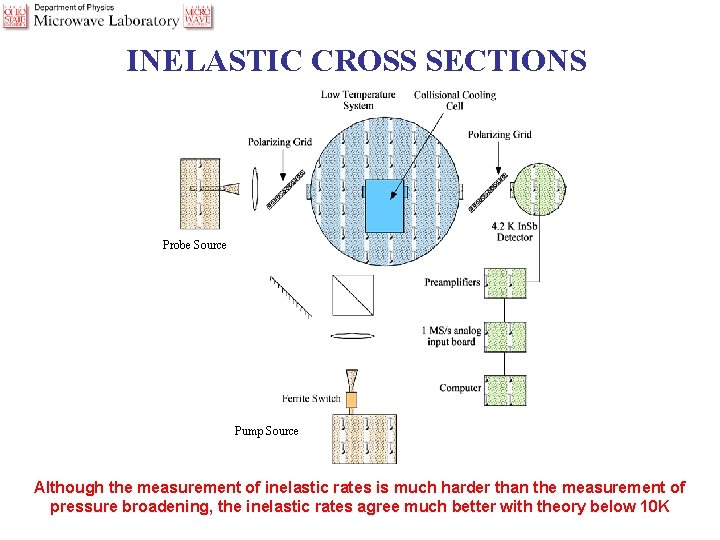
INELASTIC CROSS SECTIONS Probe Source Pump Source Although the measurement of inelastic rates is much harder than the measurement of pressure broadening, the inelastic rates agree much better with theory below 10 K

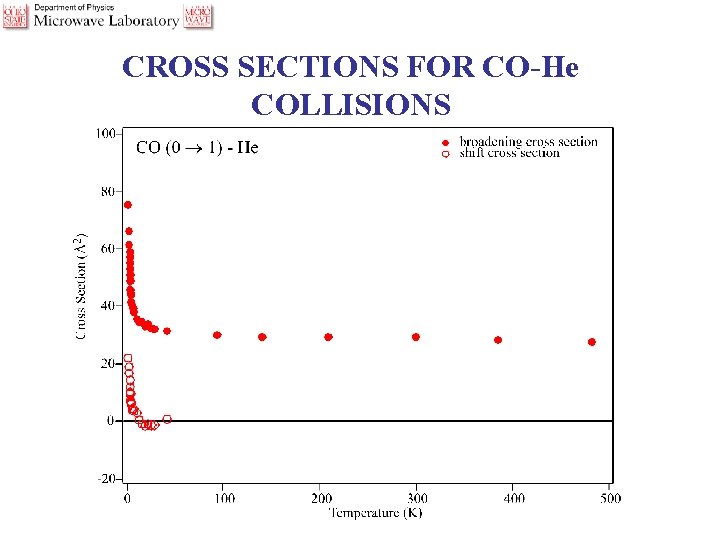
CROSS SECTIONS FOR CO-He COLLISIONS

CO-He CROSS SECTIONS

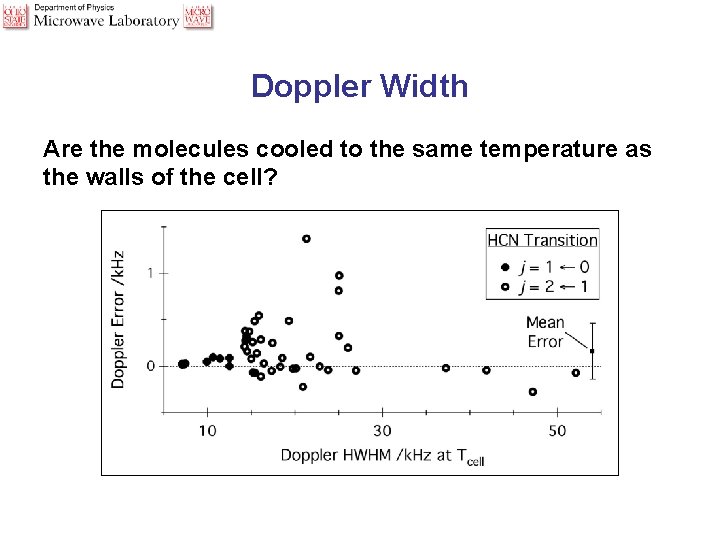
Doppler Width Are the molecules cooled to the same temperature as the walls of the cell?

HCN 1 0 Elastic Cross Section

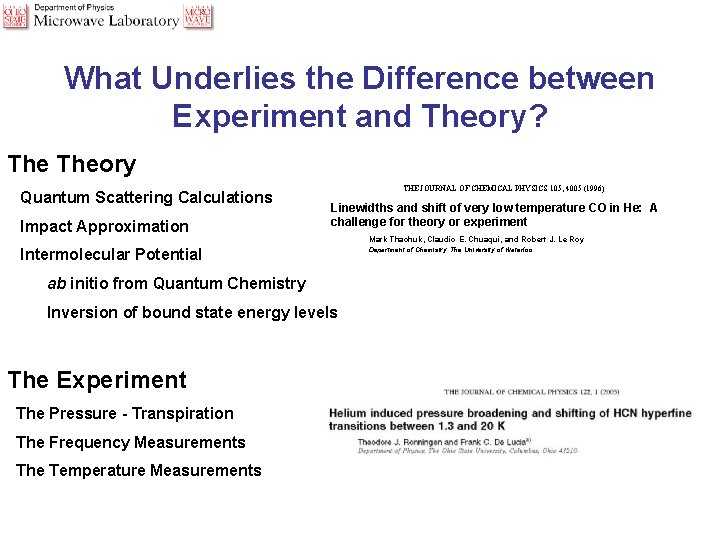
What Underlies the Difference between Experiment and Theory? Theory Quantum Scattering Calculations Impact Approximation THE JOURNAL OF CHEMICAL PHYSICS 105, 4005 (1996) Linewidths and shift of very low temperature CO in He: A challenge for theory or experiment Intermolecular Potential ab initio from Quantum Chemistry Inversion of bound state energy levels The Experiment The Pressure - Transpiration The Frequency Measurements The Temperature Measurements Mark Thachuk, Claudio E. Chuaqui, and Robert J. Le Roy Department of Chemistry, The University of Waterloo

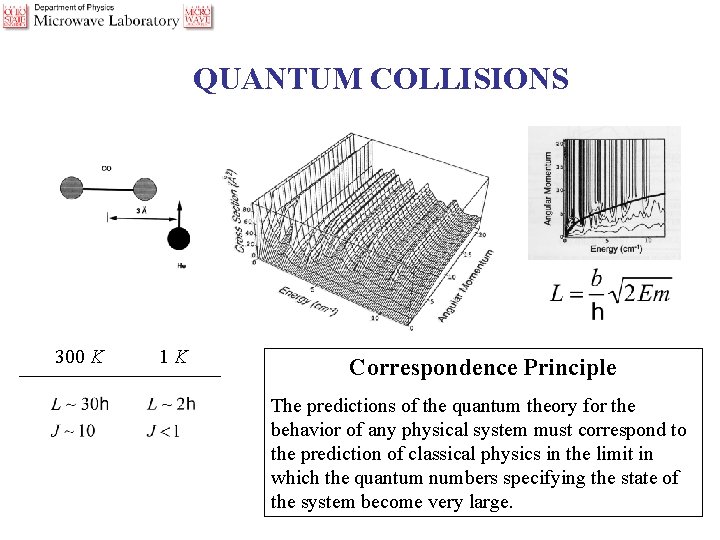
QUANTUM COLLISIONS 300 K 1 K _________________ Correspondence Principle The predictions of the quantum theory for the behavior of any physical system must correspond to the prediction of classical physics in the limit in which the quantum numbers specifying the state of the system become very large.

CH 3 Cl: SEMICLASSICAL ENERGETICS AND ANGULAR MOMENTUM

CH 3 Cl: EXPERIMENTAL SEMICLASSICAL CROSS SECTIONS Initial overpopulation of low J Relaxation to thermal population Relaxation to larger, higher J pool of states at higher temperature

Final Remarks 1. There is a very different relation between experiment and theory in collisional spectroscopy vs energy level spectroscopy. 2. This is exasperated at low temperature because of vapor pressure limits on experiment, but 3. Collisional Cooling provides an experimental method for the validation of theoretical results at low temperature. 4. Below about 10 K there gets to be a significant difference between experiment and theory (especially for the lowest J lines) for pressure broadening. 5. This difference if much less or missing for inelastic rates. 6. Is there a transition temperature above which the ‘classical averaging’ makes possible more empirical approaches?