Experimental Design Measurement of Time The SI Units

- Slides: 18

Experimental Design

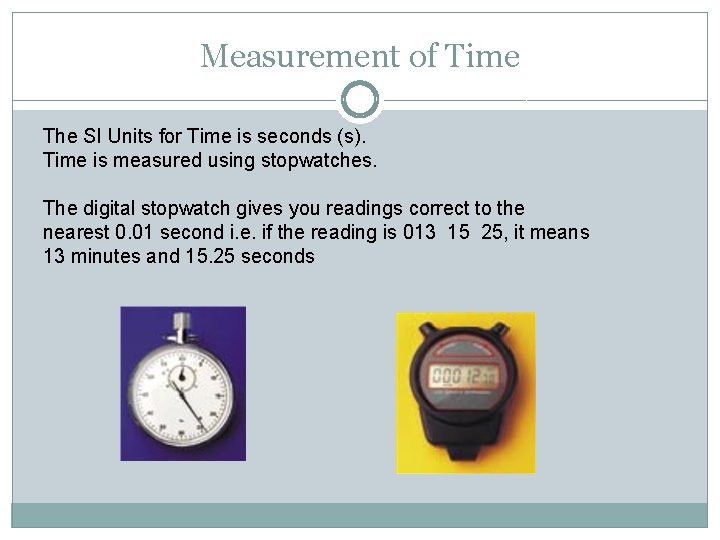
Measurement of Time The SI Units for Time is seconds (s). Time is measured using stopwatches. The digital stopwatch gives you readings correct to the nearest 0. 01 second i. e. if the reading is 013 15 25, it means 13 minutes and 15. 25 seconds

Measurement of Temperature The SI Units for Temperature is Kelvin (K) but degree Celsius (˚C) is more commonly used. Temperature can also be measured using thermometers (mercury or alcohol) or using dataloggers with temperature sensors.

Measurement of Mass The SI Units for Mass is kilograms (kg). Mass can be measured using a beam balance or an electronic balance. The electronic balance measures accurately to the nearest 0. 01 g.

Measurement of Volumes The SI Units for Volume is m 3. Measuring cylinders can measure to an accuracy of 0. 5 cm 3 or 0. 1 cm 3. (depending on the accuracy of the instrument) Conical flasks and measuring cylinders are NOT USED TO MEASURE VOLUMES OF SOLUTIONS! They are only used to store or contain solutions.

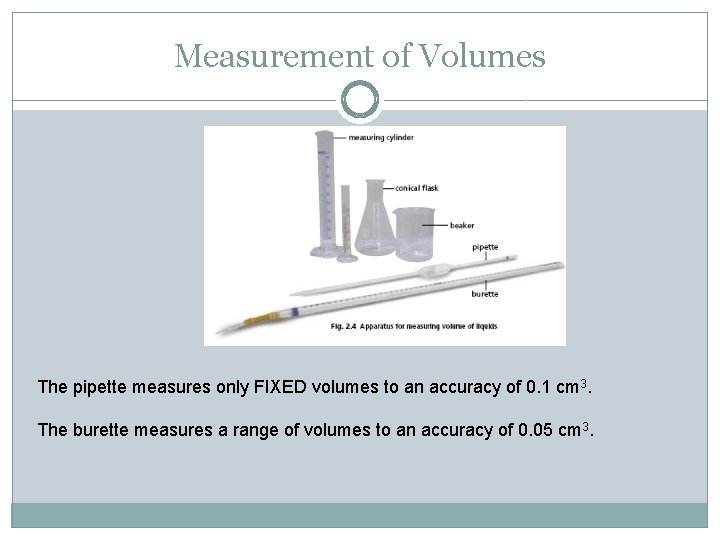
Measurement of Volumes The pipette measures only FIXED volumes to an accuracy of 0. 1 cm 3. The burette measures a range of volumes to an accuracy of 0. 05 cm 3.

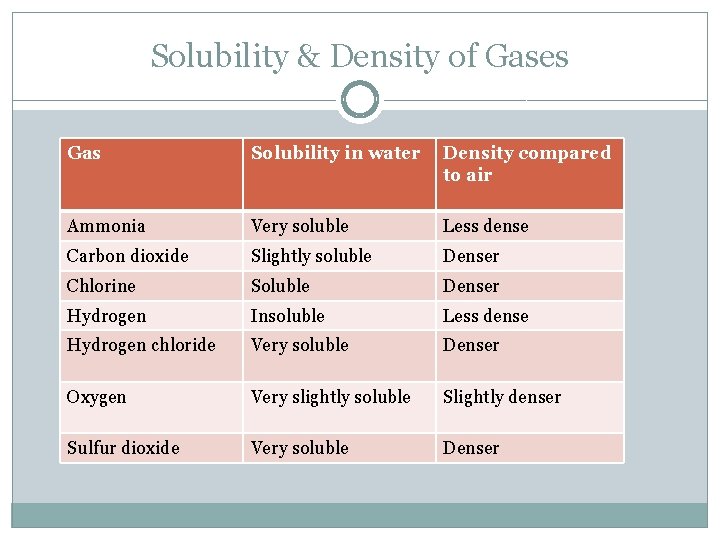
Solubility & Density of Gases Gas Solubility in water Density compared to air Ammonia Very soluble Less dense Carbon dioxide Slightly soluble Denser Chlorine Soluble Denser Hydrogen Insoluble Less dense Hydrogen chloride Very soluble Denser Oxygen Very slightly soluble Slightly denser Sulfur dioxide Very soluble Denser

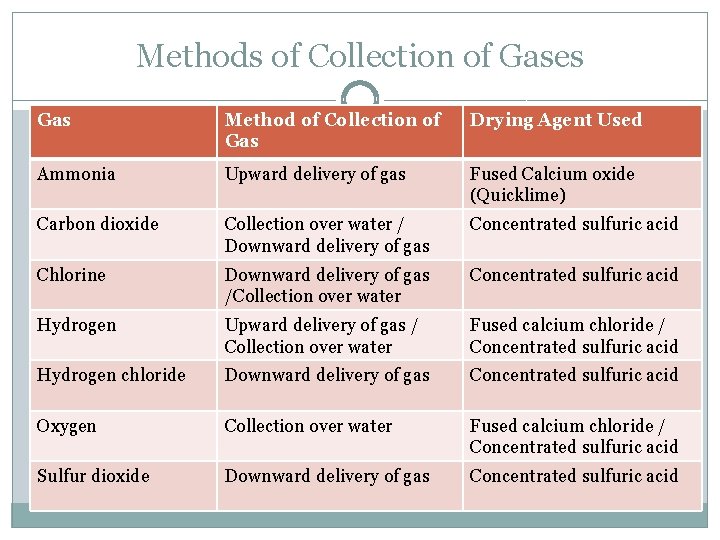
Methods of Collection of Gases Gas Method of Collection of Gas Drying Agent Used Ammonia Upward delivery of gas Fused Calcium oxide (Quicklime) Carbon dioxide Collection over water / Downward delivery of gas Concentrated sulfuric acid Chlorine Downward delivery of gas /Collection over water Concentrated sulfuric acid Hydrogen Upward delivery of gas / Collection over water Fused calcium chloride / Concentrated sulfuric acid Hydrogen chloride Downward delivery of gas Concentrated sulfuric acid Oxygen Collection over water Fused calcium chloride / Concentrated sulfuric acid Sulfur dioxide Downward delivery of gas Concentrated sulfuric acid

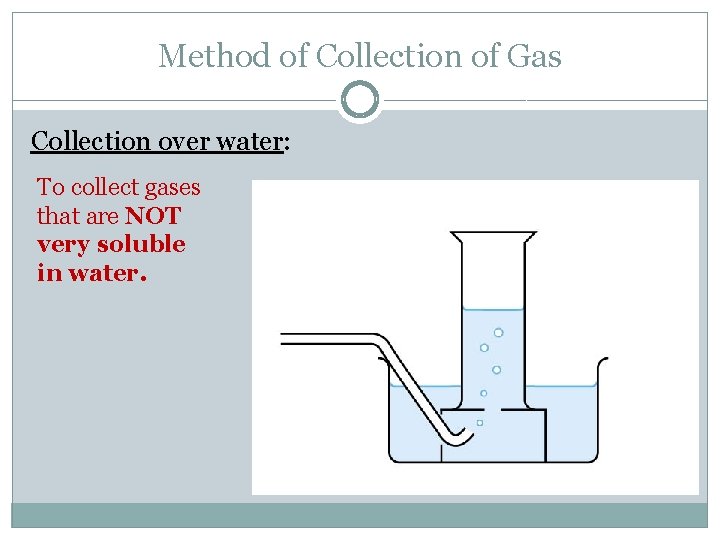
Method of Collection of Gas Collection over water: To collect gases that are NOT very soluble in water.

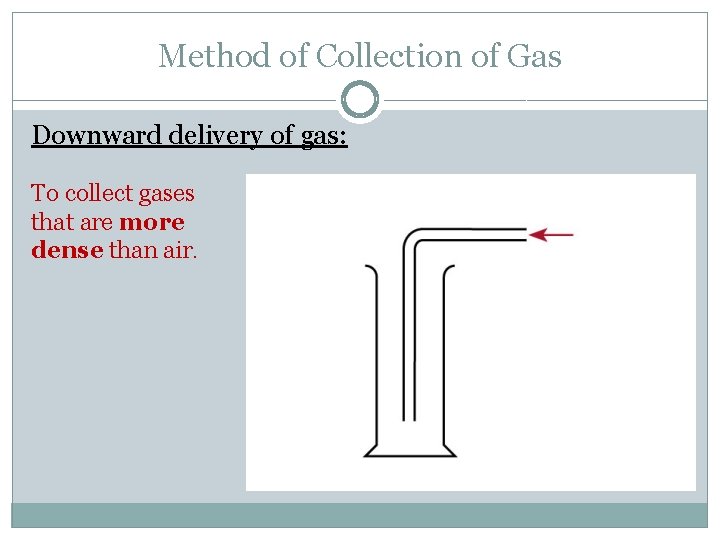
Method of Collection of Gas Downward delivery of gas: To collect gases that are more dense than air.

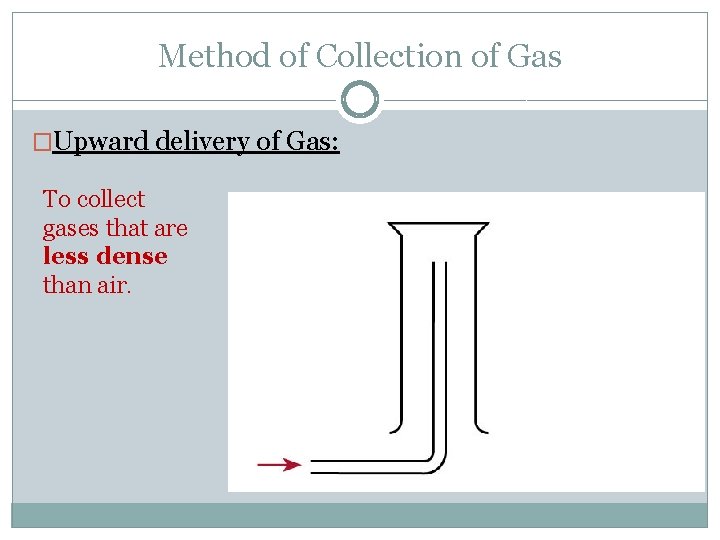
Method of Collection of Gas �Upward delivery of Gas: To collect gases that are less dense than air.

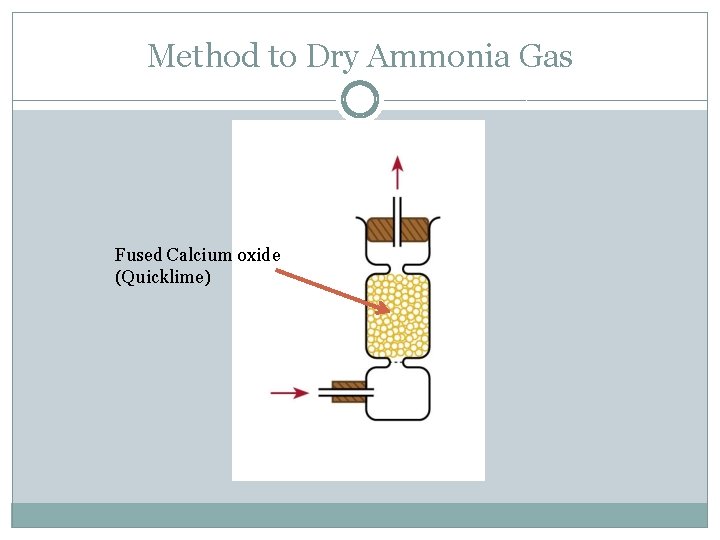
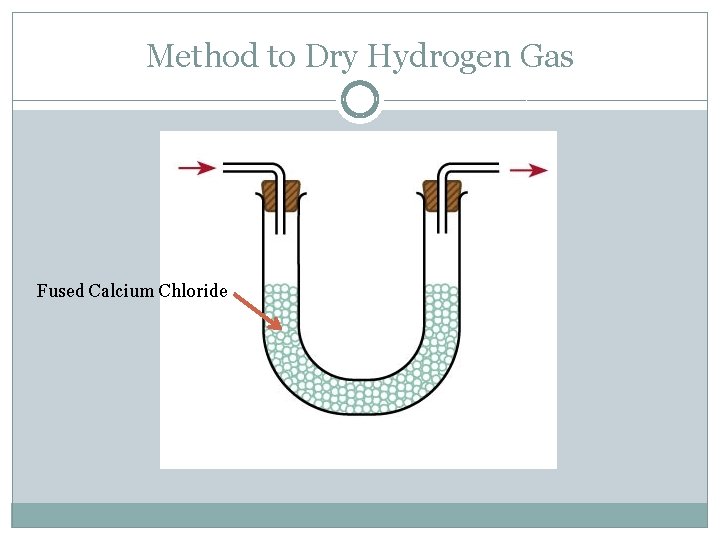
Common Drying Agents Some commonly used drying agents are concentrated sulphuric acid, quicklime (calcium oxide) and fused calcium chloride.

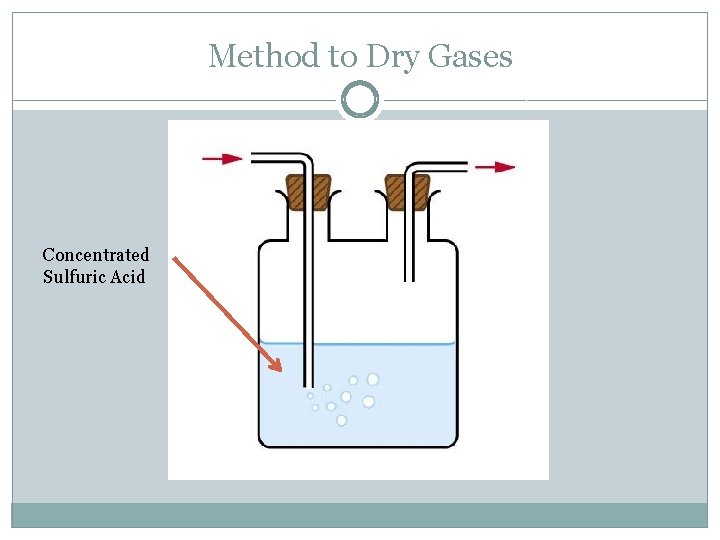
Method to Dry Gases Concentrated Sulfuric Acid

Method to Dry Ammonia Gas Fused Calcium oxide (Quicklime)

Method to Dry Hydrogen Gas Fused Calcium Chloride

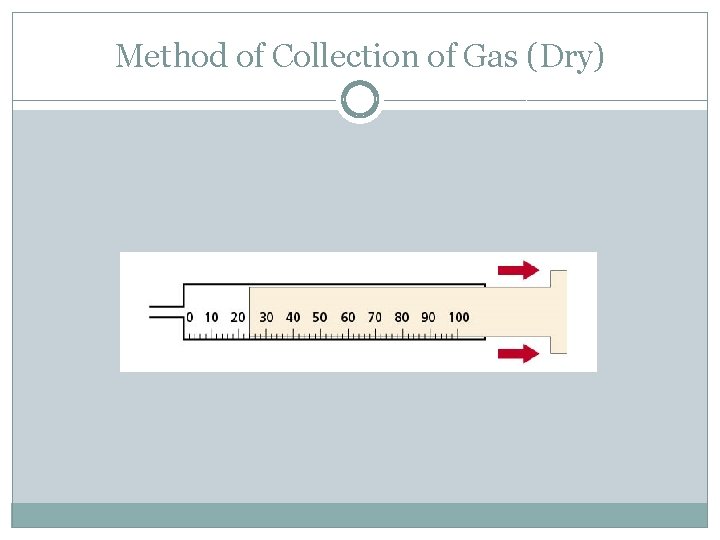
Method of Collection of Gas (Dry)

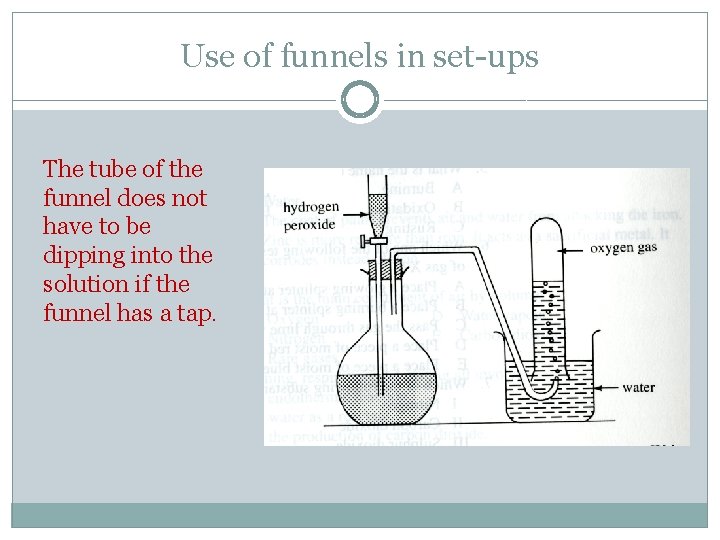
Use of funnels in set-ups The tube of the funnel does not have to be dipping into the solution if the funnel has a tap.

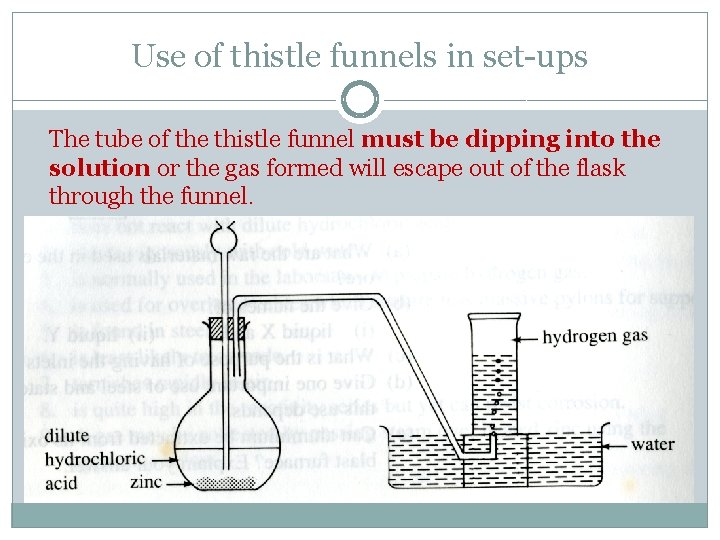
Use of thistle funnels in set-ups The tube of the thistle funnel must be dipping into the solution or the gas formed will escape out of the flask through the funnel.