Experiment20 Microscale Spectrophotometric Measurement of Iron in Foods













- Slides: 14

Experiment-20 Microscale Spectrophotometric Measurement of Iron in Foods by Standard Addition BY SIBY FRANCIS

Reagents l 2. 0 M HCl: 15 m. L/student l Hydroquinone: 4 m. L/student l Trisodium citrate dehydrate: 6 g/student l O-phenanthroline: 4 m. L/student l St. Fe (40 µg Fe/m. L): 4 m. L/student l 6 M HCl for cleaning purpose

Procedure WEAR YOUR SAFETY GOGGLES.

Procedure l Fill the clean porcelain crucible with 6 M HCl and allow to stay overnight. l Rinse the crucible with distilled water and dry it in the oven. l Dry the crucible until the weight agrees to 0. 001 g. l Get the unknown from the stockroom and add 5 -6 g of food particles in the crucible.

Procedure Cont… l Weigh the crucible again to get the exact mass of the food particles. l Heat the crucible for 3 hours. l First, at low flame to dry the food then increase the flame temp to char the sample. l After charring, use the hottest flame possible flame to ignite the black solid. l If the sample bursts into fire use the lid to smother the flames.

Procedure Cont… l Burn until we get white ash. l Cool the crucible to room temp and add 10 m. L 2. 0 M HCl to the sample. l Swirl the crucible gently l Filter the mixture through a small filter and collect the solution in a small vial. l Weigh 0. 71 g of trisodium citrate to four 10 -m. L volumetric flasks. l Using the 2 m. L Vol. pipet add 2. 00 m. L of the ash solution to each of the flasks.

Procedure Cont. . l Add 4 m. L of distilled water and swirl to dissolve the citrate. (p. H 3. 6) l Using micropipet add 0. 20 m. L of hydroquinone solution and 0. 30 m. L of phenanthroline solution to each flask. l Label the vol. Flask 0 -3 and add 0. 250 m. L, 0. 500 m. L, 0. 750 m. L St. Fe to flask 1 -3 respectively. l The flask marked Zero have 0µg/m. L of St. Fe.

Procedure (Blank prep) l Blank is prepared by mixing 0. 71 g of trisodium citrate dehydrate, 2. 00 m. L of 2. 0 M HCl, 0. 20 ml of hydroquinone solution, 0. 30 m. L of phenanthroline solution, and diluting to 10 m. L.

Using a spectrophotometer. l Measure the absorbance of each solution at 512 nm in a 1 -cm cell with dist. water in the reference. l With the help of a pasteur pipet wash the cuvet and start measuring from the blank solution in the order of increasing concentration. l Wash the cuvet with dist. water and rinse it with the solution to be used before measuring any absorbance.

WHY WE ADD? ? O-Phenanthroline Trisodium citrate Hydroquinone

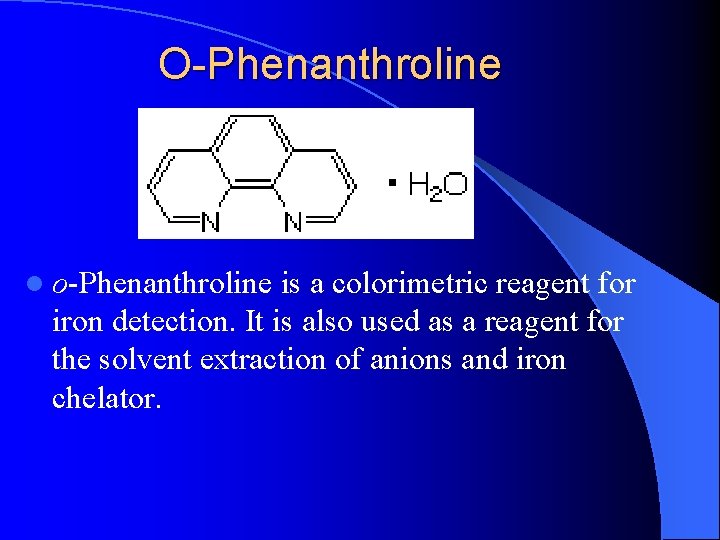
O-Phenanthroline l o-Phenanthroline is a colorimetric reagent for iron detection. It is also used as a reagent for the solvent extraction of anions and iron chelator.

O-Phenanthroline l Fe 2+ ions are colorless because it has molar absorptivity of zero for visible light. Thus, to determine the concentration of Fe 2+ ions in solution using a visible wavelength of light, one must react the ions quantitatively with something that will form colored species. In this experiment Fe 2+ ions (Lewis acids) react with o -phenanthroline molecules, C 12 H 8 N 2 , (Lewis bases) to form orange-red [Fe(o-Phen)3]2+ ions

Trisodium citrate l There is another Lewis acid in the solution - the H+ ion. These ions compete with Fe 2+ ions for the o-phenanthroline molecules. Citrate ions, C 6 H 5 O 73 -, are added to react with the hydrogen ions to form citric acid molecules and, thus, to remove them from competition.

Hydroquinone l Another complication is that Fe 2+ ions are easily oxidized to Fe 3+ ions with air. Hydroquinone, HOC 6 H 4 OH, is added to reduce any Fe 3+ back to Fe 2+ forming quinone, OC 6 H 4 O.