EXPERIMENT ONE MEASURING THE SAPONIFICATION VALUE OF CASTOR













- Slides: 23

EXPERIMENT ONE MEASURING THE SAPONIFICATION VALUE OF CASTOR OIL

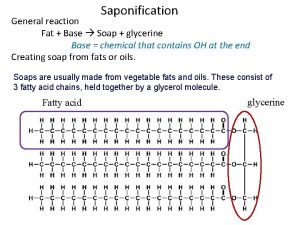
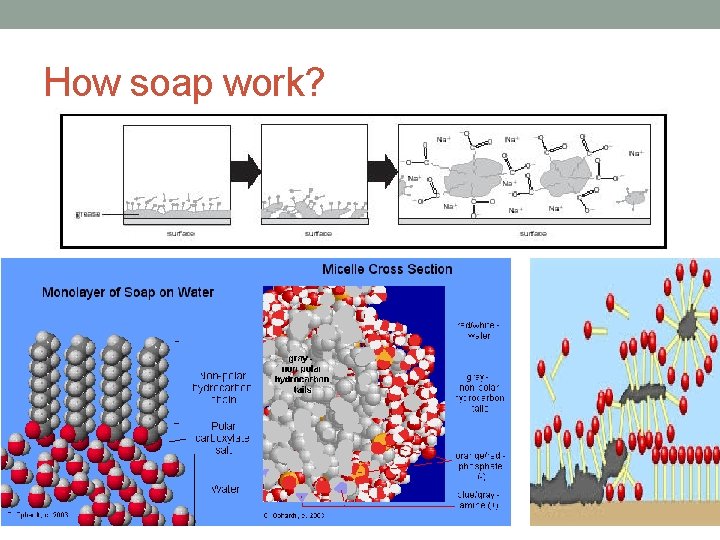
Key Concepts • Soaps are produced during the chemical reaction known as saponification. • Saponification is the reaction between a fat or oil and a base, producing glycerol and a salt (soap) fat or oil + base -----> glycerol + salt (soap) • Soaps are usually sodium or potassium salts of long-chain fatty acids. • Soaps are cleaning agents or detergents. • Molecules of soap are made up of two parts: a non-polar, hydrophobic tail consisting of a long hydrocarbon chain. a hydrophilic, negatively charged, carboxylate ion (anion) head.

Introduction: • Castor oil is a vegetable oil obtained from castor beans (Ricinus communis Euphorbiaceae), very pale yellow liquid with a boiling point of 313 °C and a density of 0. 961 g/ml.

Pharmacological Uses: • Plasticizer in food, candy and cosmetic industries. • Laxative. • Has some anti-inflammatory action.

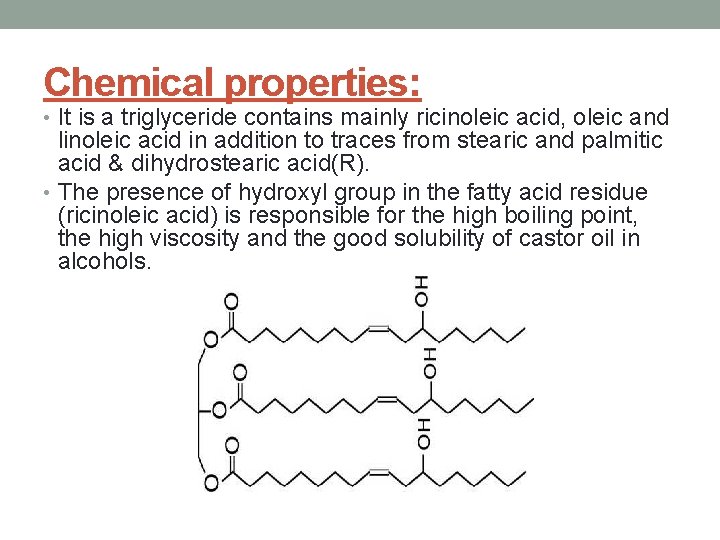
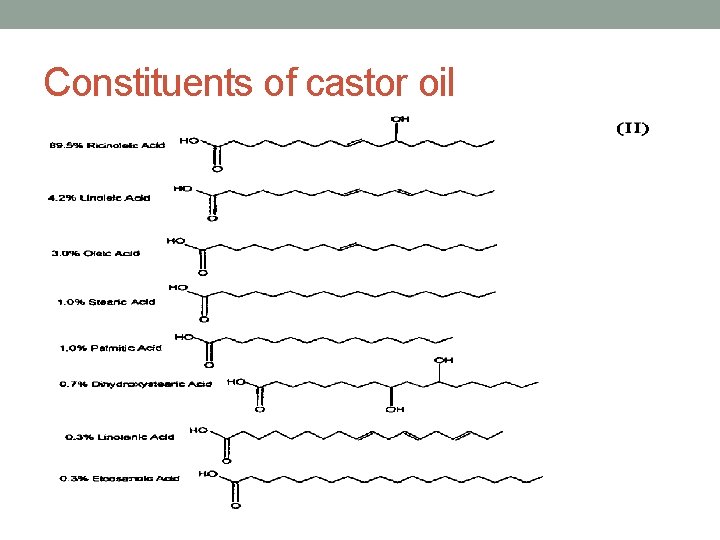
Chemical properties: • It is a triglyceride contains mainly ricinoleic acid, oleic and linoleic acid in addition to traces from stearic and palmitic acid & dihydrostearic acid(R). • The presence of hydroxyl group in the fatty acid residue (ricinoleic acid) is responsible for the high boiling point, the high viscosity and the good solubility of castor oil in alcohols.

Constituents of castor oil

Chemical properties: • Castor oil is miscible in ether, but immiscible with water.

Identification tests (1) Castor oil is soluble in 95% alcohol, miscible in anhydrous alcohol, and ether. (2) Specific gravity should be 0. 952∼ 0. 966. (3) Refractive Index [n](20, D) should be 1. 477∼ 1. 481.

Saponification value: • Is the number of milligrams of potassium hydroxide required to saponify 1 g of fat (triester) under the conditions specified.

Principle: • Oil is samplified by refluxing with a known excess of alcoholic KOH soln. • The alkali required for saponification is determined by titration of the excess KOH with standard HCl.

Assay test: • Accurately weigh 2 g of castor oil and place them in a 100 ml rounded bottom flask. • Pipette 25 ml of 0. 5 M ethanolic KOH (Mwt = 56. 1 g/mole). • Fit a reflux condenser and heat the mixture (do not forget to add boiling chips) using a water bath for one hour. • Remove from the water bath, then add four drops phenolphthalein indicator (What will be the colour of the solution? ). • Titrate with 0. 5 M HCl while the mixture still hot (Why? ). • Note: what is the colour of the end point? • Repeat the same procedure for the blank. (which contains only 25 ml of 0. 5 M KOH)

Reasons for applying heat • To accelerate the hydrolysis reaction

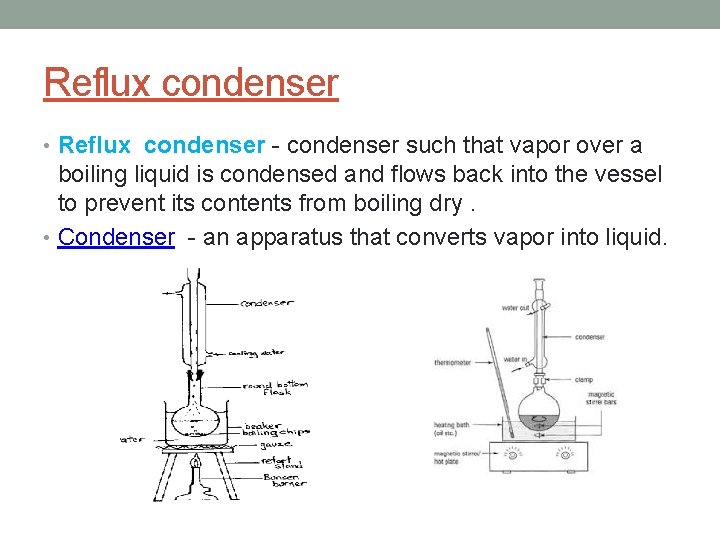
Reflux condenser • Reflux condenser - condenser such that vapor over a boiling liquid is condensed and flows back into the vessel to prevent its contents from boiling dry. • Condenser - an apparatus that converts vapor into liquid.

Reasons for using reflux • prevent evaporation of the solvent (media for reaction) • prevent sample burning. • To permit the accurate reaction & accurate result. • Constant & well defined temperature. • Controls rate of heating.

Notes: • Using a gauze over the Bunsen allows the mixture to boil more smoothly. • Anti-bumping granules A few are added to a liquid before heating and they encourage smooth boiling ( distribute heat evenly through out the liquid) & prevent overheating & bumping (violent boiling may damage the apparatus). • Four or five granules is enough to work well - students often add ten times this amount! • Grease is applied to junctions to prevent gas leakage.

Back titration (process) • A back titration, or indirect titration, is generally a two-stage analytical technique: • Reactant A of unknown concentration is reacted with excess reactant B of known concentration. • A titration is then performed to determine the amount of reactant B in excess.

Back titrations are used when: • one of the reactants is volatile, for example ammonia. • an acid or a base is an insoluble salt, for example calcium carbonate. • a particular reaction is too slow.

Phenolptalein indicator color according to changes in p. H

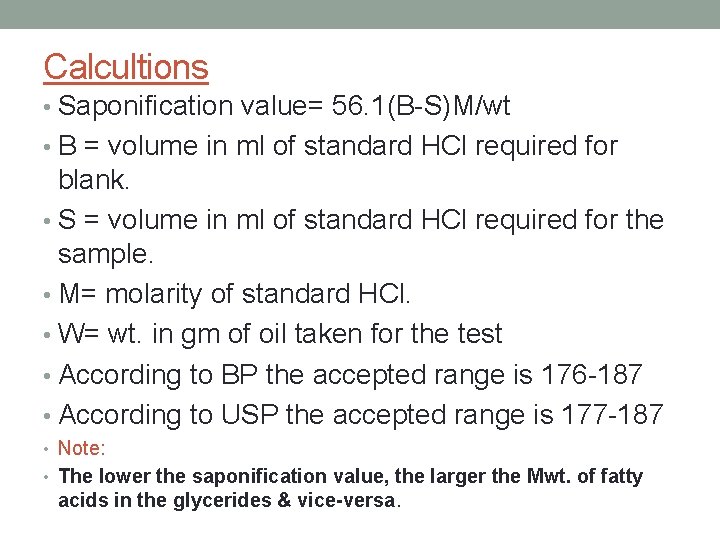
Calcultions • Saponification value= 56. 1(B-S)M/wt • B = volume in ml of standard HCl required for blank. • S = volume in ml of standard HCl required for the sample. • M= molarity of standard HCl. • W= wt. in gm of oil taken for the test • According to BP the accepted range is 176 -187 • According to USP the accepted range is 177 -187 • Note: • The lower the saponification value, the larger the Mwt. of fatty acids in the glycerides & vice-versa.

Significance of saponification value • to insure that our final soap is not caustic hot & not overly superfatted. Factors affect fluidity of soap: • Unsaturated fat • Potassium hydroxide is used

How soap work?

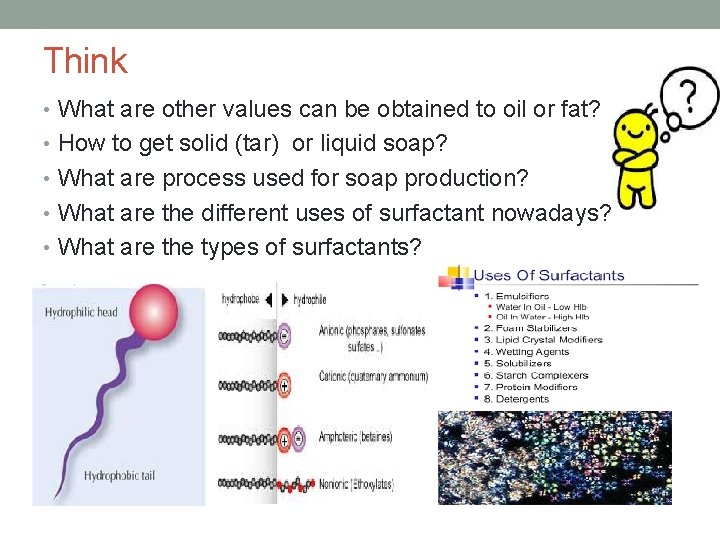
Think • What are other values can be obtained to oil or fat? • How to get solid (tar) or liquid soap? • What are process used for soap production? • What are the different uses of surfactant nowadays? • What are the types of surfactants?

Thank you for listening
 Saponification value significance
Saponification value significance Acid value and saponification value
Acid value and saponification value Saponification value definition
Saponification value definition Significance of saponification value
Significance of saponification value Saponification value
Saponification value Triglyceride molecule
Triglyceride molecule Le blaireau sans gene de yvon danet
Le blaireau sans gene de yvon danet Nido de castor
Nido de castor Castor data management
Castor data management Sirius og castor
Sirius og castor 0nilo
0nilo Christine castor
Christine castor Strategiearten
Strategiearten Castor plateforme
Castor plateforme Contoh value creation
Contoh value creation Ethyl acetate reaction with sodium hydroxide
Ethyl acetate reaction with sodium hydroxide Classes of lipids
Classes of lipids Dx/dt=k(a-x)(b-x)
Dx/dt=k(a-x)(b-x) Glycerophospholipid structure
Glycerophospholipid structure Intramolecular fischer esterification
Intramolecular fischer esterification One god one empire one religion
One god one empire one religion One one little dog run
One one little dog run One king one law one faith
One king one law one faith One empire one god one emperor
One empire one god one emperor