EXPERIMENT 9 THE PERIODIC TABLE INTRODUCTION The periodic

EXPERIMENT 9 - THE PERIODIC TABLE

INTRODUCTION: � The periodic table is arranged into seven horizontal rows and 18 vertical columns. The particular row and column tell us much about the properties of an element. � The relationship between the electronic configuration and the chemical properties of elements is most readily expressed in terms of the periodic table. The elements are arranged in the table in the order of increasing atomic numbers in horizontal rows, called periods, of such length that elements with similar properties (size) recur periodically. � The elements with similar chemical properties are arranged in vertical columns called groups (or families) and they have the same number of valence electrons. Elements found in the same horizontal row have the same number of energy levels but cannot be expected to behave in similar ways.

OBJECTIVES: � � TO EXAMINE THE PROPERTIES OF ELEMENTS IN VARIOUS GROUPS TO OBSERVE THE REACTION OF COUMPOUND S OF ELEMENTS THAT BELONG TO THE SAME GROUP IN THE PERIODIC TABLE
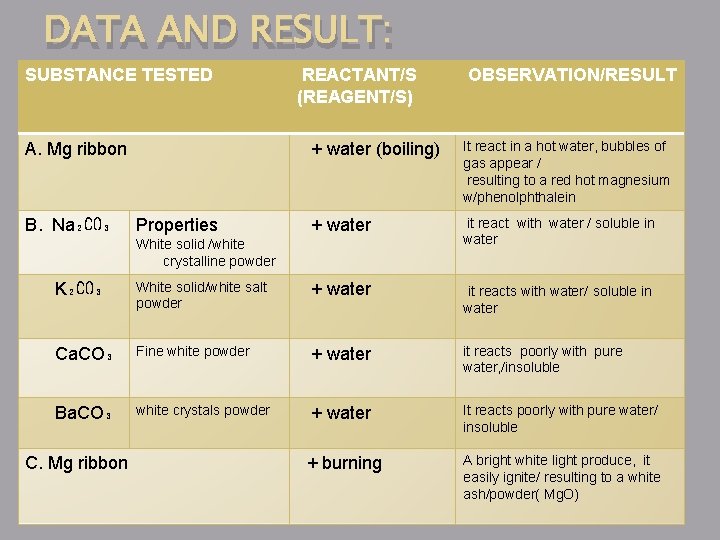
DATA AND RESULT: SUBSTANCE TESTED A. Mg ribbon B. Na₂CO₃ Properties REACTANT/S (REAGENT/S) OBSERVATION/RESULT + water (boiling) It react in a hot water, bubbles of gas appear / resulting to a red hot magnesium w/phenolphthalein + water it react with water / soluble in water White solid /white crystalline powder K₂CO₃ White solid/white salt powder + water it reacts with water/ soluble in water Ca. CO₃ Fine white powder + water it reacts poorly with pure water, /insoluble Ba. CO₃ white crystals powder + water It reacts poorly with pure water/ insoluble + burning A bright white light produce, it easily ignite/ resulting to a white ash/powder( Mg. O) C. Mg ribbon
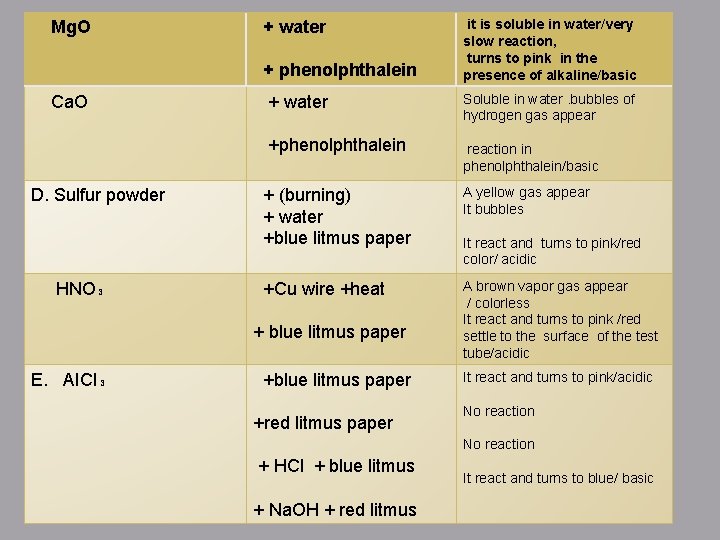
Mg. O + water + phenolphthalein Ca. O D. Sulfur powder HNO₃ + water Soluble in water. bubbles of hydrogen gas appear +phenolphthalein reaction in phenolphthalein/basic + (burning) + water +blue litmus paper A yellow gas appear It bubbles +Cu wire +heat A brown vapor gas appear / colorless It react and turns to pink /red settle to the surface of the test tube/acidic + blue litmus paper E. AICI₃ it is soluble in water/very slow reaction, turns to pink in the presence of alkaline/basic +blue litmus paper +red litmus paper It react and turns to pink/red color/ acidic It react and turns to pink/acidic No reaction + HCl + blue litmus + Na. OH + red litmus It react and turns to blue/ basic

Reaction of Active Metals with H₂O Group I- A metal + water→ Base + Hydrogen ions Group I } 2 Na + H₂O → 2 Na. OH + H₂↑ 2 K + H₂O→ 2 KOH + H₂↑ ( more reactive because rxn occurs at RT only) � Group II- metal+ water→ Base Mg + O₂→ Mg. O heat Group 2 – Mg. O + H₂O → Mg[OH]₂ + H₂↑ ( occurs at high boiling temp) turns to fuschia solution w/ phenolphthalein �
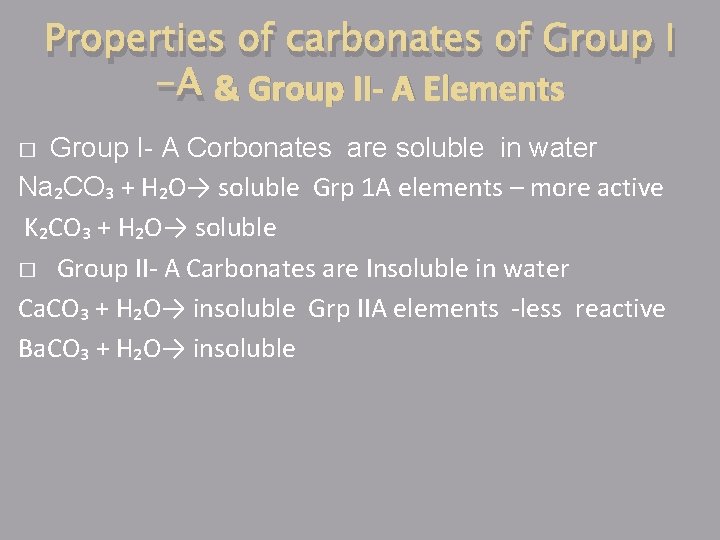
Properties of carbonates of Group I -A & Group II- A Elements Group I- A Corbonates are soluble in water Na₂CO₃ + H₂O→ soluble Grp 1 A elements – more active K₂CO₃ + H₂O→ soluble � Group II- A Carbonates are Insoluble in water Ca. CO₃ + H₂O→ insoluble Grp IIA elements -less reactive Ba. CO₃ + H₂O→ insoluble �

Properties of Metal Oxides Mg. O Mg +O →Mg. O +H₂O →Mg(OH)₂ +H₂↑+phenolphthalein ↓ Colorless to fuschia pink - base � � � Ca. O + H 2 O → Ca(OH)2 + H₂↑ phenolphthalein ↓ Fuschia pink- base
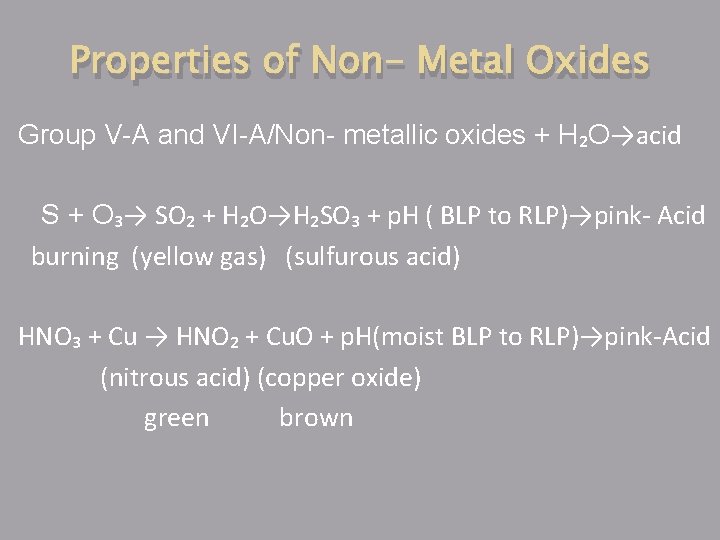
Properties of Non- Metal Oxides Group V-A and VI-A/Non- metallic oxides + H₂O→acid S + O₃→ SO₂ + H₂O→H₂SO₃ + p. H ( BLP to RLP)→pink- Acid burning (yellow gas) (sulfurous acid) HNO₃ + Cu → HNO₂ + Cu. O + p. H(moist BLP to RLP)→pink-Acid (nitrous acid) (copper oxide) green brown

Metalloid Property AICI₃ + 6 H₂O→ AI(H₂O)₆⁺³ + 3 C Hydrated aluminum ion AI(H₂O)₆⁺³ + H₂O→ AI(H₂O)₅OH⁺² + H³O Acid Base Acid (Hydrated (hydrated hydranium aluminum ions ) Aluminum hydroxide ) AICI₃→BLP- acidic + HCI → - Acidic AICI₃→RLP- no change + HCI → -Acidic (acidic) �
CONCLUSION: � Therefore the elements in the periodic table is arranged sequence to their increasing atomic number , which arranged in rows and columns so that the elements with similar chemical properties are in the same column and said to be in the same group that have the same number of valence electron.

� In our experiment, we observed and determined the reaction of the elements that belongs in the same group in the periodic table. Some chemicals react to one another and some did not.

GROUP 3 � � � � � TAMANG, FREMMA AQUINO, DARLY JONNAH LAMAYAN, DARTIA TAVAKKOLI, REYHANEY BAUTISTA, CARLO GAVIOLA, ALAIN SENA, ZYRUS DEHGNAN, SINA ADEL, JERSON
- Slides: 13