Experiment 9 The Gran Plot Calculations Presented by

Experiment 9: The Gran Plot - Calculations Presented by Alfred Tadalan
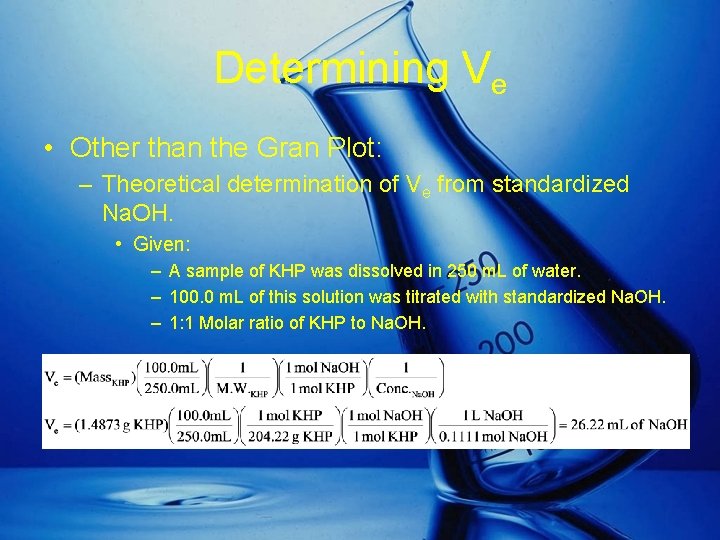
Determining Ve • Other than the Gran Plot: – Theoretical determination of Ve from standardized Na. OH. • Given: – A sample of KHP was dissolved in 250 m. L of water. – 100. 0 m. L of this solution was titrated with standardized Na. OH. – 1: 1 Molar ratio of KHP to Na. OH.

Determining Ve (cont. ) • Other than the Gran Plot (cont. ): – Colored Indicator (Phenolphthalein). • Ve = 25. 36 m. L. – Plot of p. H versus VNa. OH.
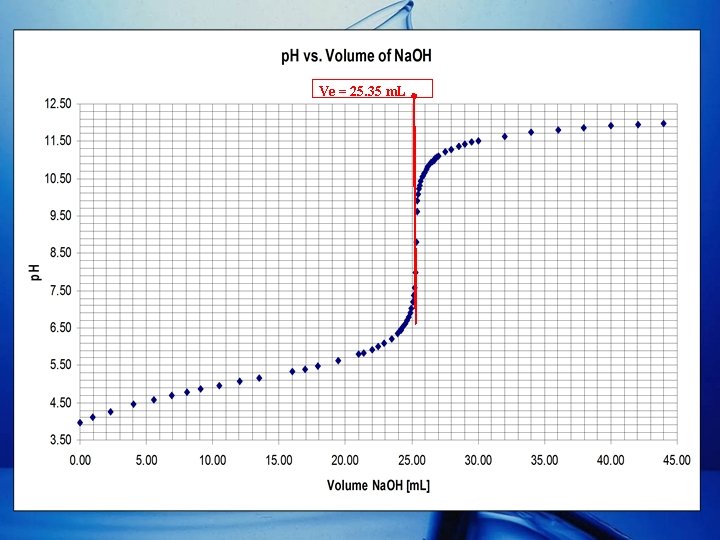
Ve = 25. 35 m. L

Determining Ve (cont. ) • Other than the Gran Plot (cont. ): – Plot of VNa. OH vs. 2 nd Derivative.
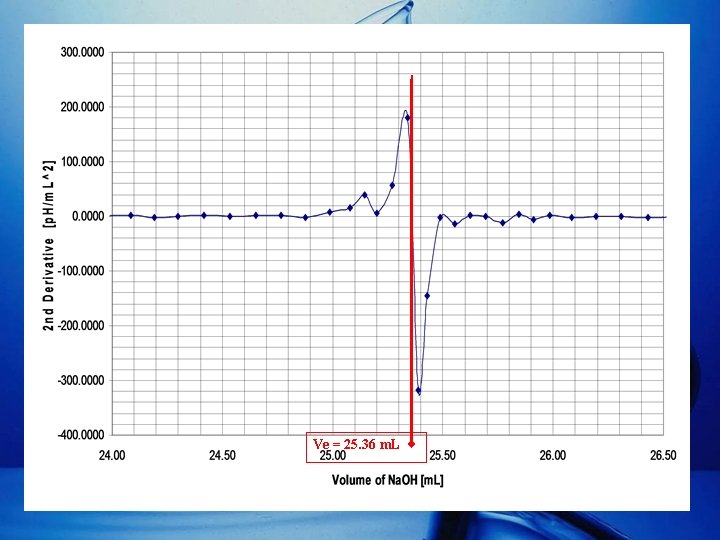
Ve = 25. 36 m. L

Detemination of Ve via Gran Plot • Why use Gran Plot? – Derivatives are less accurate near the end point. – Buffering is minimal. – Electrode response is sluggish.

Detemination of Ve via Gran Plot (cont. ) • The Gran Plot is a graph of VNa. OH vs. VNa. OH 10 -p. H • The Gran plot utilizes the data points between Ve and 0. 9 Ve. – Ve = 25. 36 m. L. – 0. 9 Ve = 22. 82 m. L.
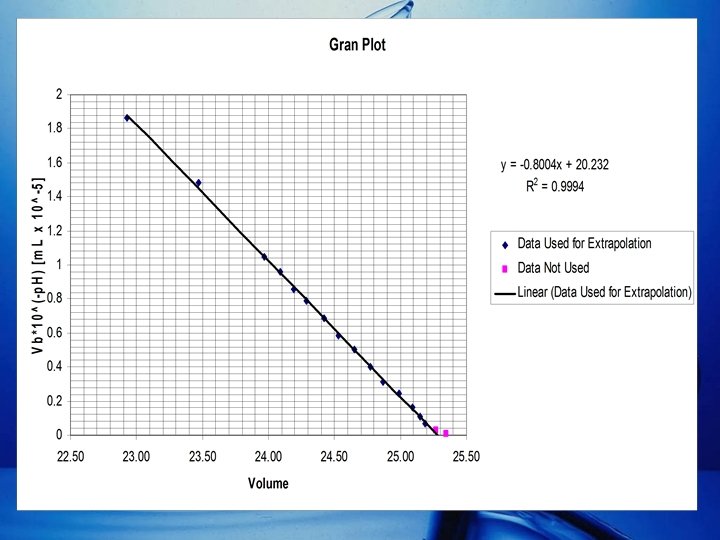
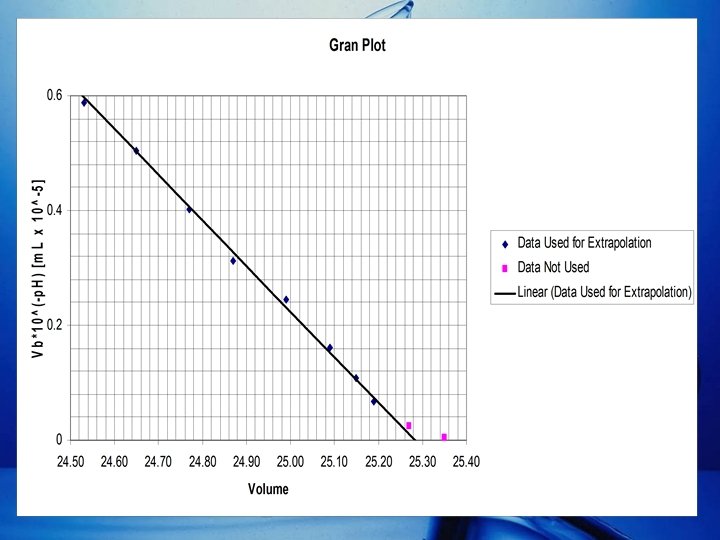

Detemination of Ve via Gran Plot (cont. ) • Because of the inaccuracies near the end point, only the linear portion of the Gran Plot is interpolated to determine Ve. • The equation of the line is determined by the Least-Squares Equations. • Where: – m = Slope – b = y-intercept – x = VNa. OH – y = VNa. OH 10 -p. H

Detemination of Ve via Gran Plot (cont. ) • Equation of the Linear portion of the Gran Plot: y = (-8. 004(10 -6))x + 2. 023(10 -4) VNa. OH 10 -p. H= (-8. 004(10 -6))(VNa. OH) + 2. 023(10 -4) • Ve is the VNa. OH at the x-intercept of the Gran Plot. 0 = (-8. 004(10 -6))(Ve) + 2. 023(10 -4) Ve =25. 27 m. L
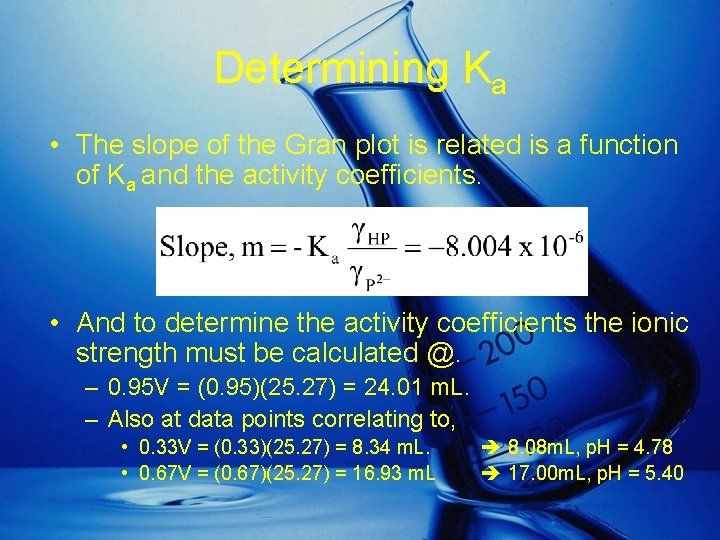
Determining Ka • The slope of the Gran plot is related is a function of Ka and the activity coefficients. • And to determine the activity coefficients the ionic strength must be calculated @. – 0. 95 V = (0. 95)(25. 27) = 24. 01 m. L. – Also at data points correlating to, • 0. 33 V = (0. 33)(25. 27) = 8. 34 m. L. • 0. 67 V = (0. 67)(25. 27) = 16. 93 m. L 8. 08 m. L, p. H = 4. 78 17. 00 m. L, p. H = 5. 40

Determining Ionic Strength • The concentration of each ion in solution is calculated.

Determining Ionic Strength (cont. ) • Ionic Strength: • Using the ionic strength, Table 8 -1 can be referenced to determine the activity coefficients for HP- and P 2 -
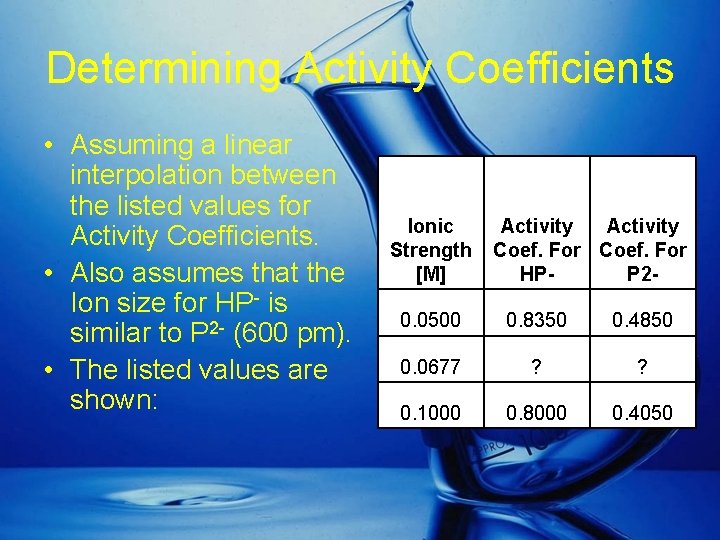
Determining Activity Coefficients • Assuming a linear interpolation between the listed values for Activity Coefficients. • Also assumes that the Ion size for HP- is similar to P 2 - (600 pm). • The listed values are shown: Ionic Strength [M] Activity Coef. For HPP 2 - 0. 0500 0. 8350 0. 4850 0. 0677 ? ? 0. 1000 0. 8000 0. 4050
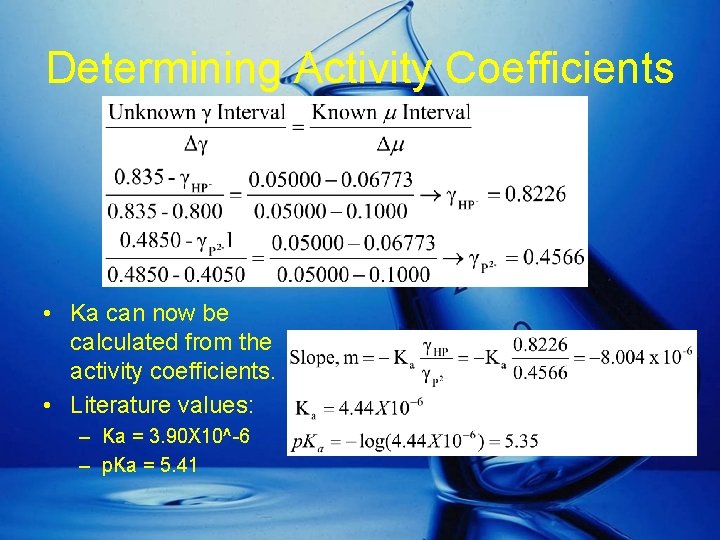
Determining Activity Coefficients • Ka can now be calculated from the activity coefficients. • Literature values: – Ka = 3. 90 X 10^-6 – p. Ka = 5. 41
- Slides: 17