Experiment 7 Preparation and Properties of Buffers Purposes

Experiment 7 Preparation and Properties of Buffers Purposes 1. Grasp the properties of buffer solutions and effect factors of buffer capacity 2. Learn the preparation of buffer solution and operation of the measuring pipet 3. Learn to determine the p. H with colorimetry and p. H-meter
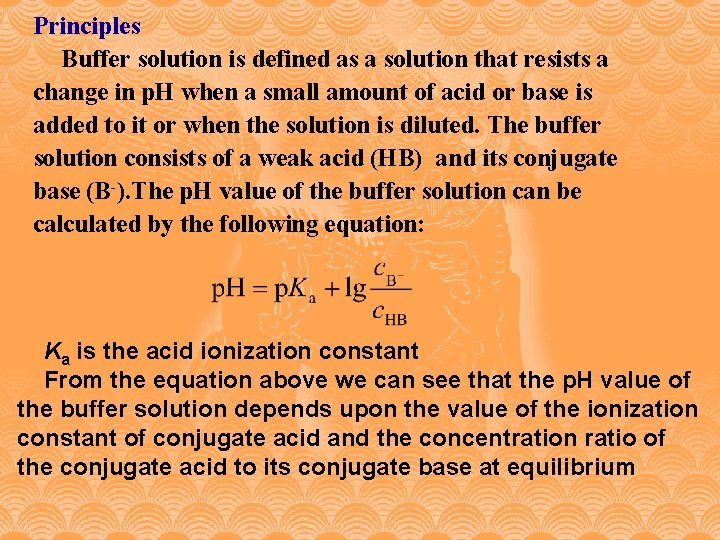
Principles Buffer solution is defined as a solution that resists a change in p. H when a small amount of acid or base is added to it or when the solution is diluted. The buffer solution consists of a weak acid (HB) and its conjugate base (B-). The p. H value of the buffer solution can be calculated by the following equation: Ka is the acid ionization constant From the equation above we can see that the p. H value of the buffer solution depends upon the value of the ionization constant of conjugate acid and the concentration ratio of the conjugate acid to its conjugate base at equilibrium
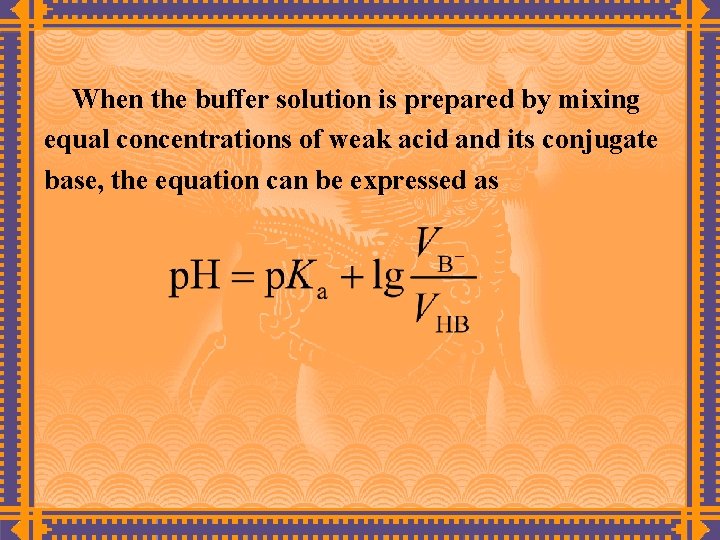
When the buffer solution is prepared by mixing equal concentrations of weak acid and its conjugate base, the equation can be expressed as
Buffer capacity is a quantitative measure of the ability of the buffer solution to resist changes in p. H, it depends on concentrations and the ratio of [B]/[HB]
p. H value of a sample solution can be measured by p. H-meter. There are two electrodes on a p. H-meter, one is reference electrode which electrode potential is a constant in some conditions; Another electrode is sensitive to [H+]. When they are immersed in a solution, the potential difference between the two electrodes is related to p. H of the solution. The p. H meter is ordinarily used when an accurate determination of p. H is needed.

3. Procedure (1) Preparation of a buffer solution Calculate the volumes of 0. 1 mol·L-1 HAc solution and 0. 1 mol·L-1 Na. Ac solution (p. Ka=4. 75) required for preparing 30 m. L of a p. H=4 buffer solution. According to the results of calculation, add the HAc and Na. Ac solution with buret into a small beaker, mix the solution well. Measure its p. H value with p. H indicator paper.

(2) Properties of buffer solutions Measure out the solutions according to Table, determine its p. H value after adding acid, base and distilled water, account for the reasons.
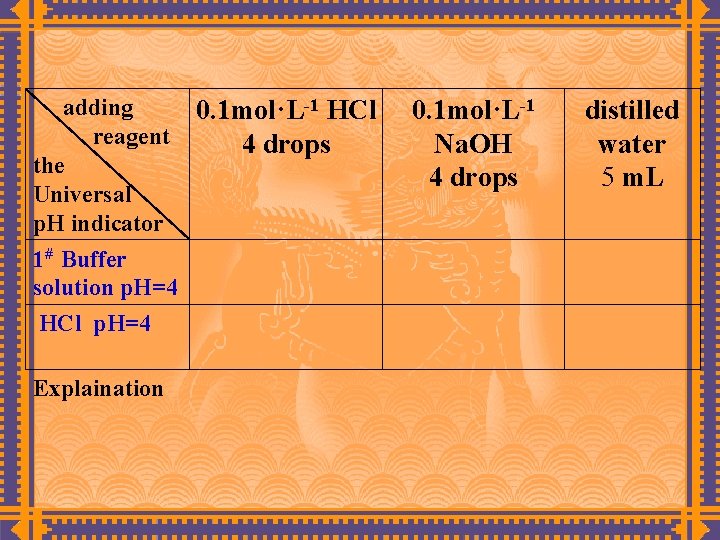
adding reagent the Universal p. H indicator 1# Buffer solution p. H=4 HCl p. H=4 Explaination 0. 1 mol·L-1 HCl 4 drops 0. 1 mol·L-1 Na. OH 4 drops distilled water 5 m. L
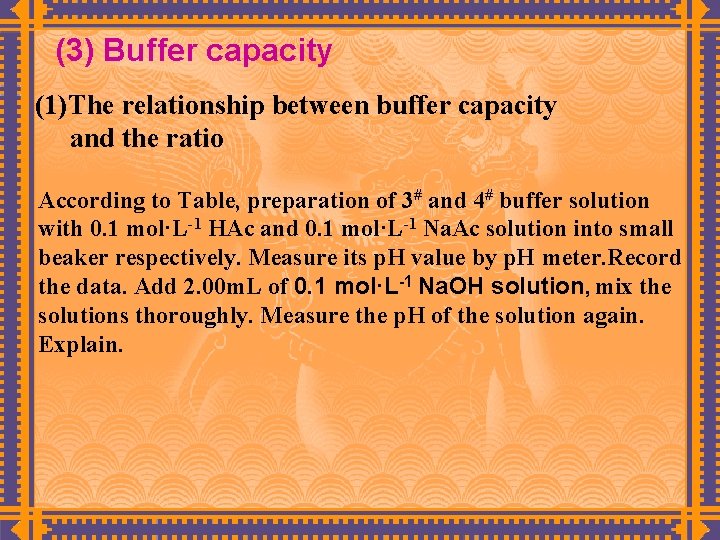
(3) Buffer capacity (1)The relationship between buffer capacity and the ratio According to Table, preparation of 3# and 4# buffer solution with 0. 1 mol·L-1 HAc and 0. 1 mol·L-1 Na. Ac solution into small beaker respectively. Measure its p. H value by p. H meter. Record the data. Add 2. 00 m. L of 0. 1 mol·L-1 Na. OH solution, mix the solutions thoroughly. Measure the p. H of the solution again. Explain.
- Slides: 9