Experiment 6 Kinetic Study of inversion of cane

![Average [ α ] = Plot a graph of concentration (c) versus angle of Average [ α ] = Plot a graph of concentration (c) versus angle of](https://slidetodoc.com/presentation_image/5c794d2a7b432595a15bbbb7fb591e7f/image-9.jpg)



- Slides: 16

Experiment (6) : Kinetic Study of inversion of cane sugar catalyzed by an acid Theory In chemistry, specific rotation ([α]) is a property of a chiral chemical compound. It is defined as the change in orientation of monochromatic plane-polarized light, per unit distance–concentration product. as the light passes through a sample of a compound in solution




PART - I A solution of sucrose is optically active. It is dextro-rotatory. Fructose is laevo-rotatory. Glucose is dextro-rotatory, and the mixture of products has the resultant laevo-rotatory. The angle of rotation depends on : (a) Temperature. (b) wavelength of light used. (c) length of the liquid column through which the light passes. (d) nature of the substrate.

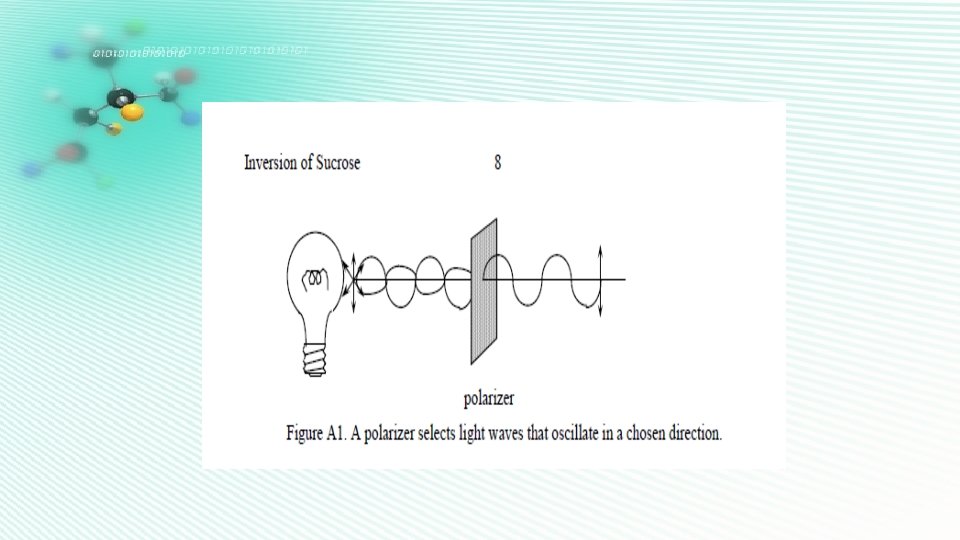
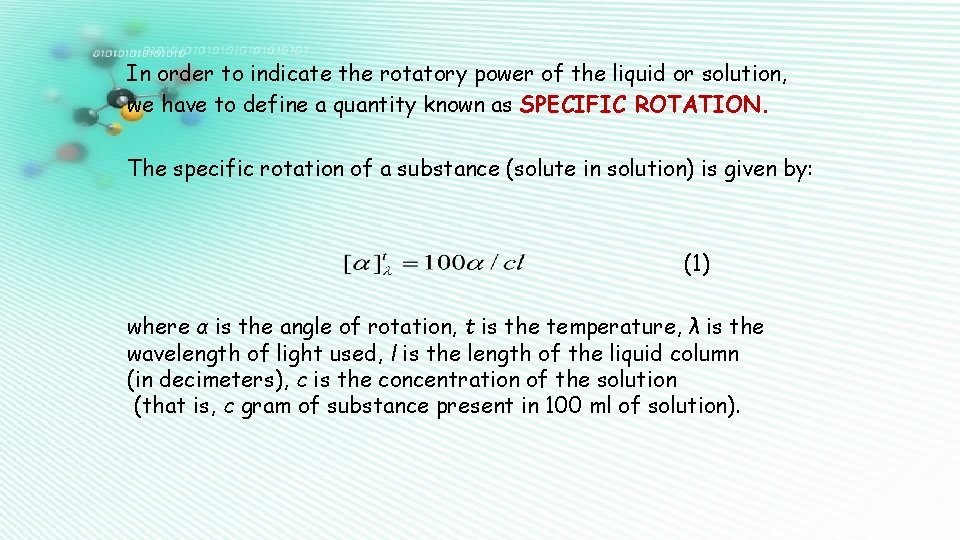
In order to indicate the rotatory power of the liquid or solution, we have to define a quantity known as SPECIFIC ROTATION. The specific rotation of a substance (solute in solution) is given by: (1) where α is the angle of rotation, t is the temperature, λ is the wavelength of light used, l is the length of the liquid column (in decimeters), c is the concentration of the solution (that is, c gram of substance present in 100 ml of solution).

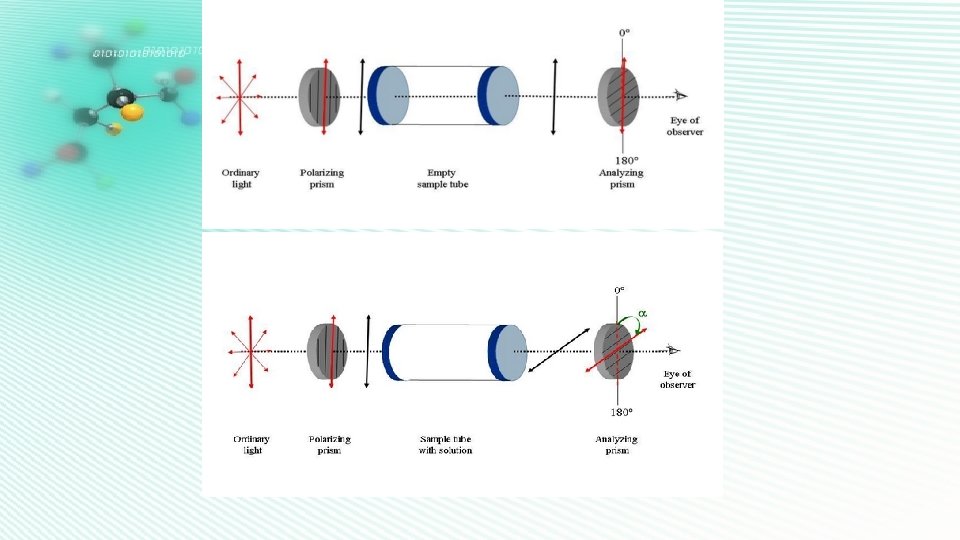
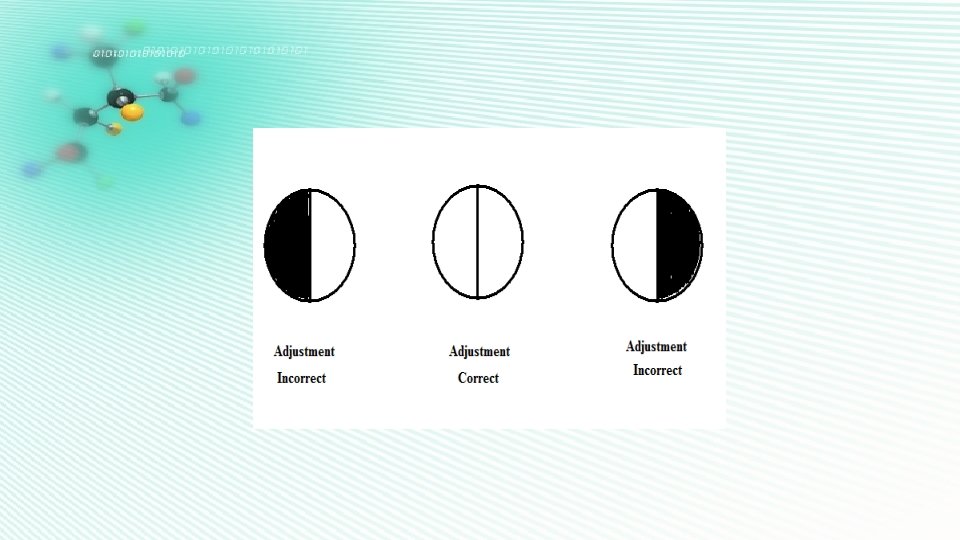
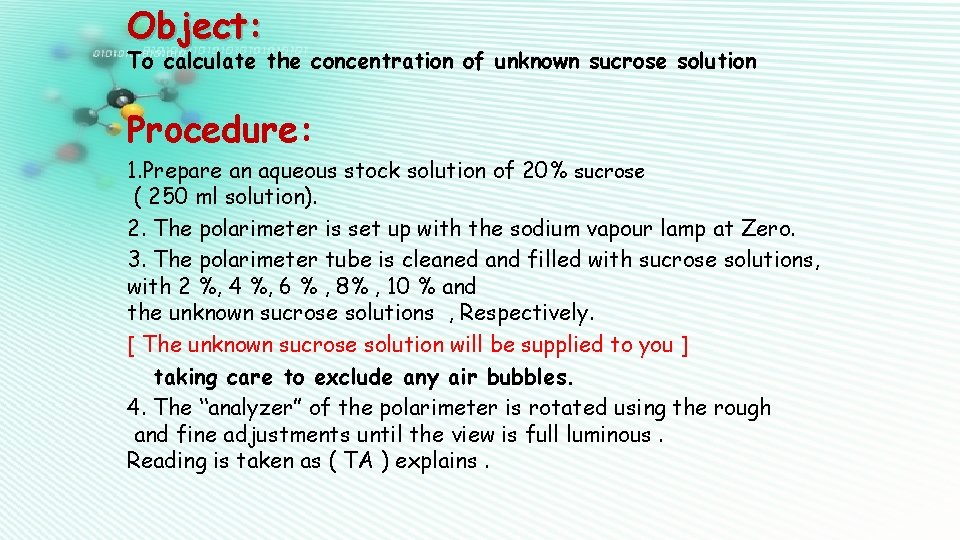
Object: To calculate the concentration of unknown sucrose solution Procedure: 1. Prepare an aqueous stock solution of 20% sucrose ( 250 ml solution). 2. The polarimeter is set up with the sodium vapour lamp at Zero. 3. The polarimeter tube is cleaned and filled with sucrose solutions, with 2 %, 4 %, 6 % , 8% , 10 % and the unknown sucrose solutions , Respectively. [ The unknown sucrose solution will be supplied to you ] taking care to exclude any air bubbles. 4. The “analyzer” of the polarimeter is rotated using the rough and fine adjustments until the view is full luminous. Reading is taken as ( TA ) explains.

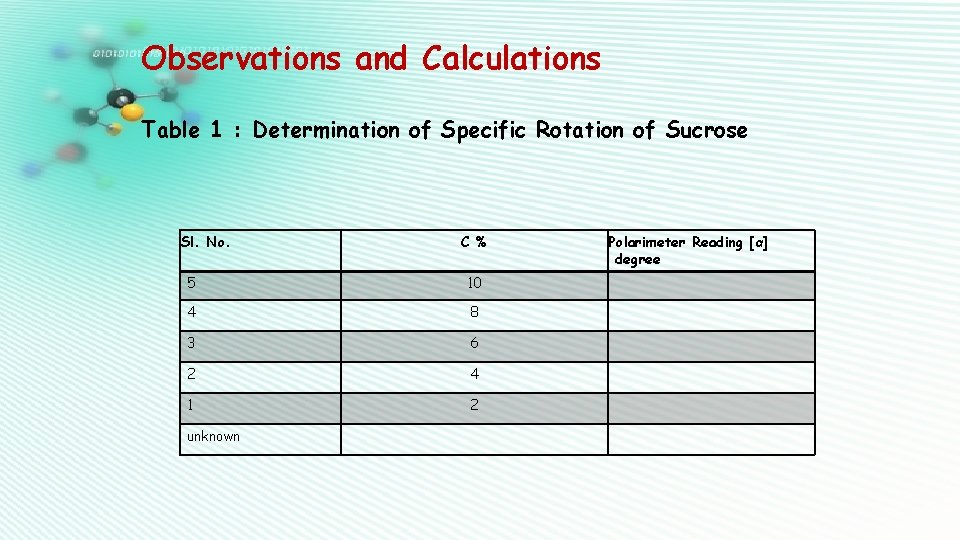
Observations and Calculations Table 1 : Determination of Specific Rotation of Sucrose Sl. No. C % 5 10 4 8 3 6 2 4 1 2 unknown Polarimeter Reading [α] degree
![Average α Plot a graph of concentration c versus angle of Average [ α ] = Plot a graph of concentration (c) versus angle of](https://slidetodoc.com/presentation_image/5c794d2a7b432595a15bbbb7fb591e7f/image-9.jpg)
Average [ α ] = Plot a graph of concentration (c) versus angle of rotation (α ). Slope = [α] l / 100. Hence, [α] = slope 100 l From the rotation of the unknown solution, the concentration of the unknown solution can be determined. Results 1. Specific Rotation of Cane Sugar at …. ° C = …… degree 2. Concentration of Unknown Solution = ………… %

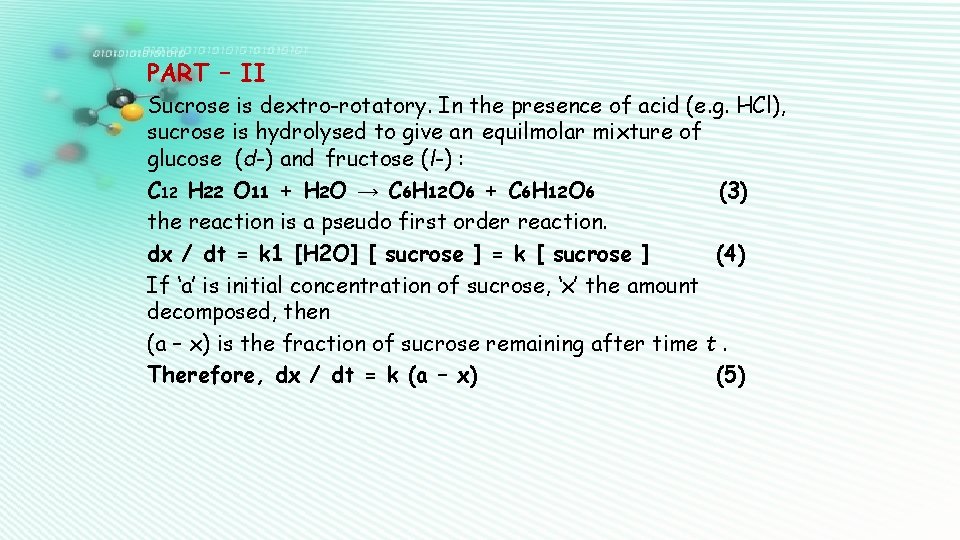
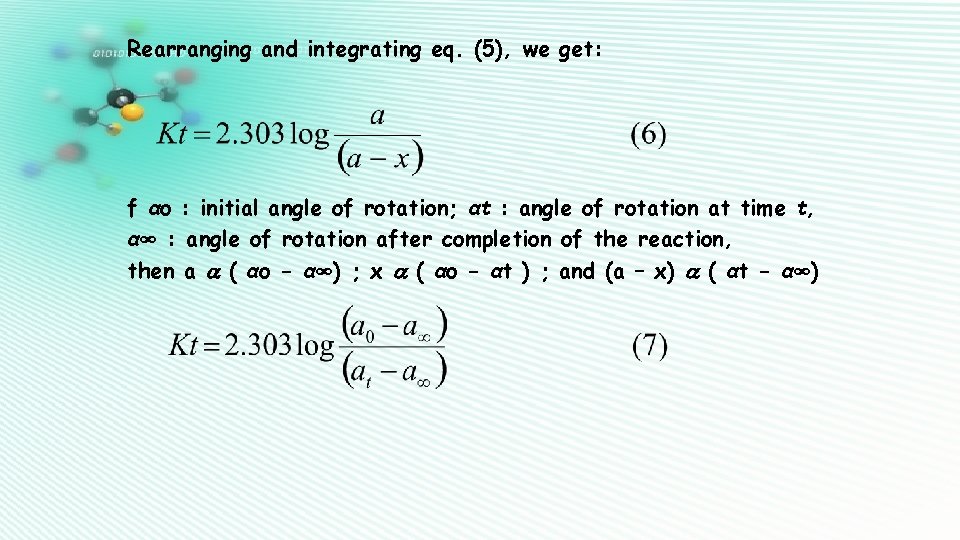
PART – II Sucrose is dextro-rotatory. In the presence of acid (e. g. HCl), sucrose is hydrolysed to give an equilmolar mixture of glucose (d-) and fructose (l-) : C 12 H 22 O 11 + H 2 O → C 6 H 12 O 6 + C 6 H 12 O 6 (3) the reaction is a pseudo first order reaction. dx / dt = k 1 [H 2 O] [ sucrose ] = k [ sucrose ] (4) If ‘a’ is initial concentration of sucrose, ‘x’ the amount decomposed, then (a – x) is the fraction of sucrose remaining after time t. Therefore, dx / dt = k (a – x) (5)

Rearranging and integrating eq. (5), we get: f αo : initial angle of rotation; αt : angle of rotation at time t, α∞ : angle of rotation after completion of the reaction, then a ( αo - α∞) ; x ( αo - αt ) ; and (a – x) ( αt - α∞)



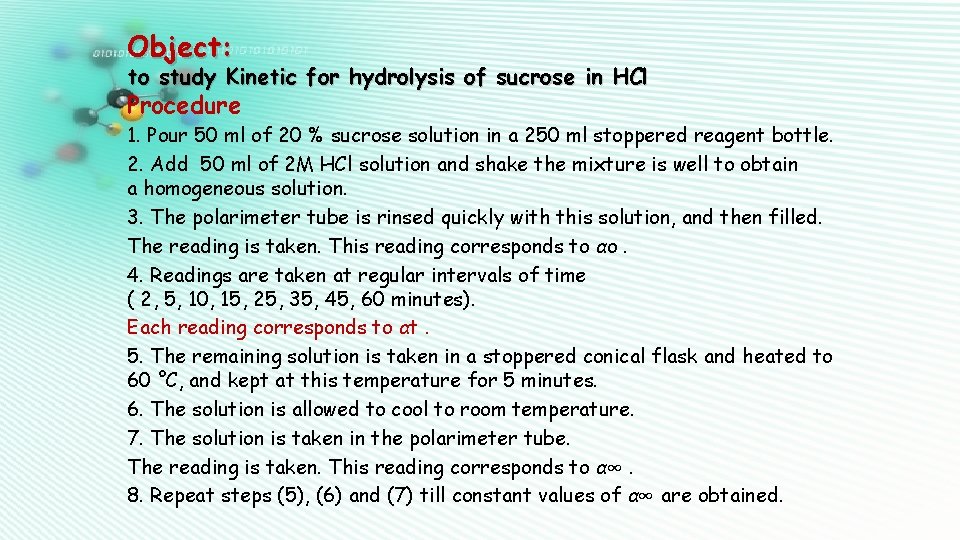
Object: to study Kinetic for hydrolysis of sucrose in HCl Procedure 1. Pour 50 ml of 20 % sucrose solution in a 250 ml stoppered reagent bottle. 2. Add 50 ml of 2 M HCl solution and shake the mixture is well to obtain a homogeneous solution. 3. The polarimeter tube is rinsed quickly with this solution, and then filled. The reading is taken. This reading corresponds to αo. 4. Readings are taken at regular intervals of time ( 2, 5, 10, 15, 25, 35, 45, 60 minutes). Each reading corresponds to αt. 5. The remaining solution is taken in a stoppered conical flask and heated to 60 °C, and kept at this temperature for 5 minutes. 6. The solution is allowed to cool to room temperature. 7. The solution is taken in the polarimeter tube. The reading is taken. This reading corresponds to α∞. 8. Repeat steps (5), (6) and (7) till constant values of α∞ are obtained.

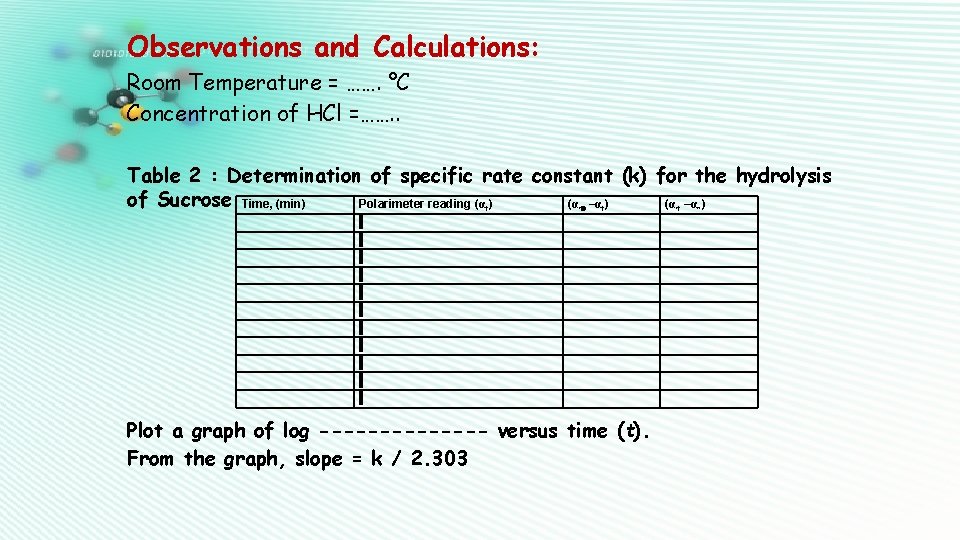
Observations and Calculations: Room Temperature = ……. °C Concentration of HCl =……. . Table 2 : Determination of specific rate constant (k) for the hydrolysis of Sucrose Time, (min) Polarimeter reading (α ) (α –α ) t Plot a graph of log ------- versus time (t). From the graph, slope = k / 2. 303 t ∞

Results 1. Specific Rotation of Sucrose at ……. °C = …… degrees 2. Rate Constant (k) for the hydrolysis of sucrose, in the presence of 1 M HCl, at ……. °C = ……………… min-1. Graphical value of k at ……. °C = ……………… min-1. •