Experiment 20 Titration of Acids and Bases CHE

Experiment 20 Titration of Acids and Bases CHE 118 1

In Lab • Work in groups of 4 • 2 people for Part A; standardization of the prepared solution of Na. OH • 2 people for Part B; analysis of an unknown acid • Each student does their own calculations after sharing data CHE 118 2

Part A • Na. OH solution is already prepared, 0. 1 M • Do not boil any water • Standardize the Na. OH solution to find the next significant figure for molarity 0. 1? M (If you use the 0. 001 g balance, then you will have 3 SF for the molarity, 0. 1? ? M) CHE 118 3

Part B • Do not boil any water • There is no weighing bottle; use weighing paper • Use the molarity determined in Part A for calculations in Part B CHE 118 4

Parts A and B • Buret readings have 2 SF after the decimal point 14. 18 m. L • Obtain volume by difference for titrant (final – initial) CHE 118 5

You. Tube Videos How to Titrate • NAIT Chemical Technology Laboratory Techniques • Carolina. Biologicals • Both are good videos on how to do a titration CHE 118 6
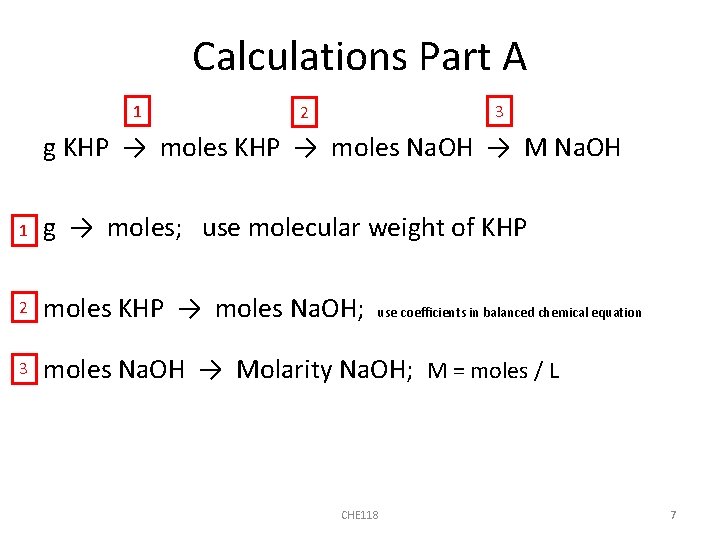
Calculations Part A 1 3 2 g KHP → moles Na. OH → M Na. OH 1 g → moles; use molecular weight of KHP 2 moles KHP → moles Na. OH; 3 moles Na. OH → Molarity Na. OH; M = moles / L use coefficients in balanced chemical equation CHE 118 7
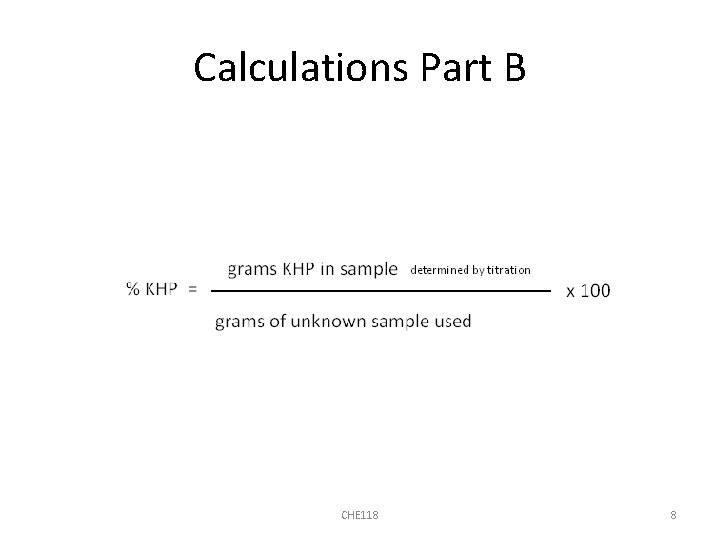
Calculations Part B CHE 118 8
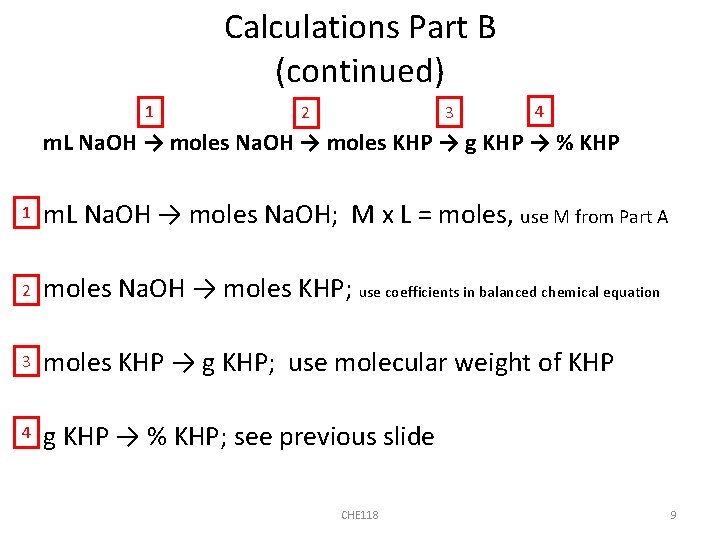
Calculations Part B (continued) 1 3 2 4 m. L Na. OH → moles KHP → g KHP → % KHP 1 m. L Na. OH → moles Na. OH; M x L = moles, use M from Part A 2 moles Na. OH → moles KHP; use coefficients in balanced chemical equation 3 moles KHP → g KHP; use molecular weight of KHP 4 g KHP → % KHP; see previous slide CHE 118 9
- Slides: 9