Experiment 2 Gravimetric Determination of Moisture in Fertilizer






![Sources � � � [1] Day R. A. Sr. and Underwood A. L. , Sources � � � [1] Day R. A. Sr. and Underwood A. L. ,](https://slidetodoc.com/presentation_image_h2/135fd75e1d26ab2902dc22c87def1fb5/image-21.jpg)
- Slides: 21

Experiment 2: Gravimetric Determination of Moisture in Fertilizer Samples By: Arvin Ramores and Jamaica Zoleta

Importance of Moisture Determination in Fertilizer Samples Moisture content is one of the most commonly measured properties of fertilizers. It is important to customers, manufacturers, regulatory officials, formulators, and farmers for a number of different reasons:

◦ (1) there are legal limits to the maximum or minimum amount of water that must be present in certain types of fertilizer ◦ (2)the grade and quality of many fertilizers depends on the amount of water they contain and ◦ (3) significant product quality issues will arise if moisture levels in fertilizers exceed manufacturer recommended levels, one example being the degradation of the product itself

Gravimetric Methods of Analysis �These are methods which are based upon the measurement of mass and therefore must be performed with high accuracy and careful measurement so not to deviate too far from the real value and to be able to come up with consistent and reliable information.

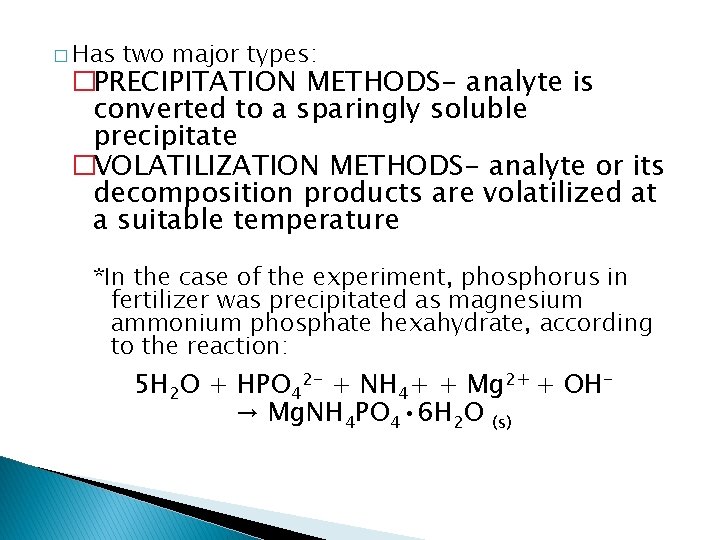
� Has two major types: �PRECIPITATION METHODS- analyte is converted to a sparingly soluble precipitate �VOLATILIZATION METHODS- analyte or its decomposition products are volatilized at a suitable temperature *In the case of the experiment, phosphorus in fertilizer was precipitated as magnesium ammonium phosphate hexahydrate, according to the reaction: 5 H 2 O + HPO 42 - + NH 4+ + Mg 2+ + OH→ Mg. NH 4 PO 4 • 6 H 2 O (s)

Different Methods for Moisture Determination: � In order to determine moisture content in samples the following methods can be performed: ◦ Common Methods of drying samples include: -Air drying- samples can be dried sufficiently for analytical determination without resort to high temperatures -Oven Drying- samples are dried in an oven at 100 -110℃. -Ignition-done at higher temperatures in order to decompose the solid into a preferred compound

Different Methods for Moisture Determination: ◦ Chemical Methods such as the Karl Fischer method -this makes use of the Karl Fischer reagent which is composed iodine, sulfur dioxide, pyridine, and methanol ◦ Microwave drying ◦ Halogen drying ◦ Infrared drying ◦ Spectroscopic methods such as infrared spectroscopy, microwave spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy can also be alternative methods

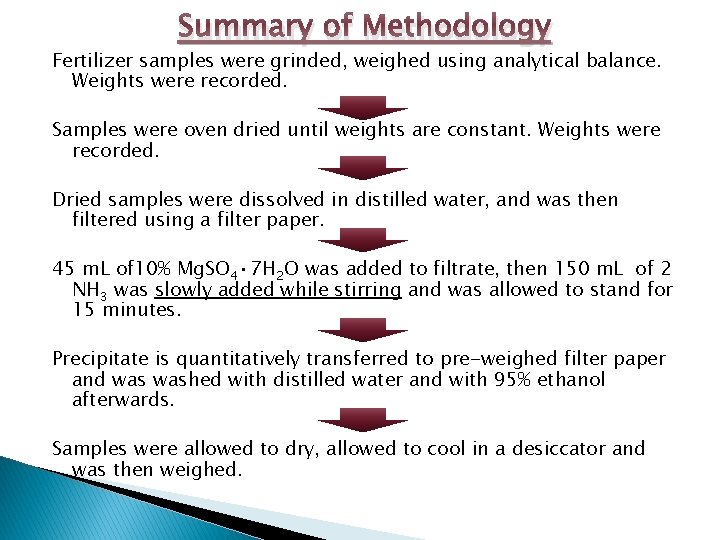
Summary of Methodology Fertilizer samples were grinded, weighed using analytical balance. Weights were recorded. Samples were oven dried until weights are constant. Weights were recorded. Dried samples were dissolved in distilled water, and was then filtered using a filter paper. 45 m. L of 10% Mg. SO 4 • 7 H 2 O was added to filtrate, then 150 m. L of 2 NH 3 was slowly added while stirring and was allowed to stand for 15 minutes. Precipitate is quantitatively transferred to pre-weighed filter paper and washed with distilled water and with 95% ethanol afterwards. Samples were allowed to dry, allowed to cool in a desiccator and was then weighed.

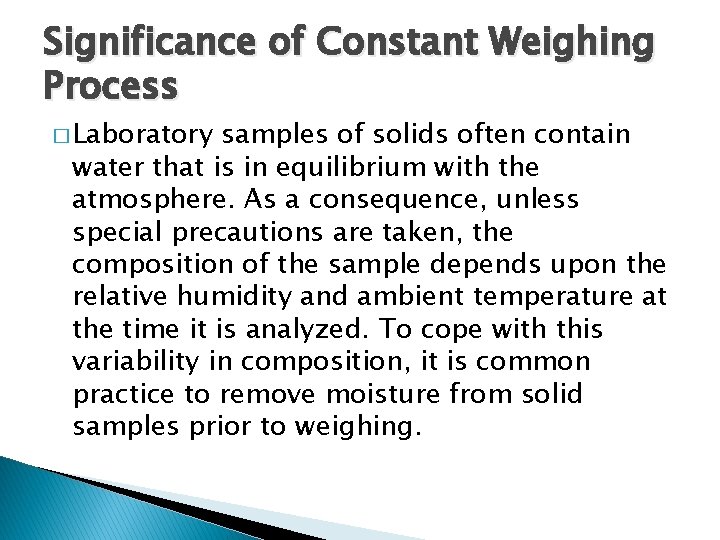
Significance of Constant Weighing Process � Laboratory samples of solids often contain water that is in equilibrium with the atmosphere. As a consequence, unless special precautions are taken, the composition of the sample depends upon the relative humidity and ambient temperature at the time it is analyzed. To cope with this variability in composition, it is common practice to remove moisture from solid samples prior to weighing.

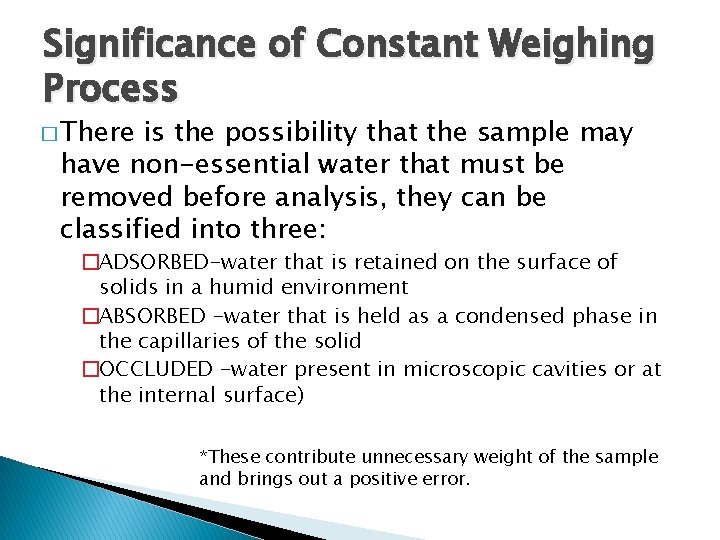
Significance of Constant Weighing Process � There is the possibility that the sample may have non-essential water that must be removed before analysis, they can be classified into three: �ADSORBED-water that is retained on the surface of solids in a humid environment �ABSORBED -water that is held as a condensed phase in the capillaries of the solid �OCCLUDED -water present in microscopic cavities or at the internal surface) *These contribute unnecessary weight of the sample and brings out a positive error.

Why use Desiccators? � Majority of solids have a certain affinity for water, and may absorb moisture from the laboratory air. Why is there a need to water always keep Reagents that readily pick up are termed hygroscopic. Thoseinthat absorb so much water the samples a desiccator that they will dissolve in it and form a especially in between weighings? concentrated solution are called deliquescent. � These types of substances will continually increase in weight while exposed to the air, that is why care must be taken to not expose the samples to air and be cooled in desiccators.

Why are the Samples Allowed to Cool in Desicators? Is it really necessary to wait for 15 -30 minutes after heating the samples in the oven before weighing in the analytical ◦balance? In the first place the body will be buoyed up by � It is highly important that the object weighed have the same temperature as the balance; if there is a temperature difference serious errors may occur. If the object has a temperature slightly higher than that of the balance, it’s apparent weight will be less than its true weight for either of both reasons: convection currents set up by itself; ◦ also if the body be cavernous, especially if its closed, the air within it may have a slightly lower density than air in the balance case, and therefore its weight will appear to be slightly less that it really is.

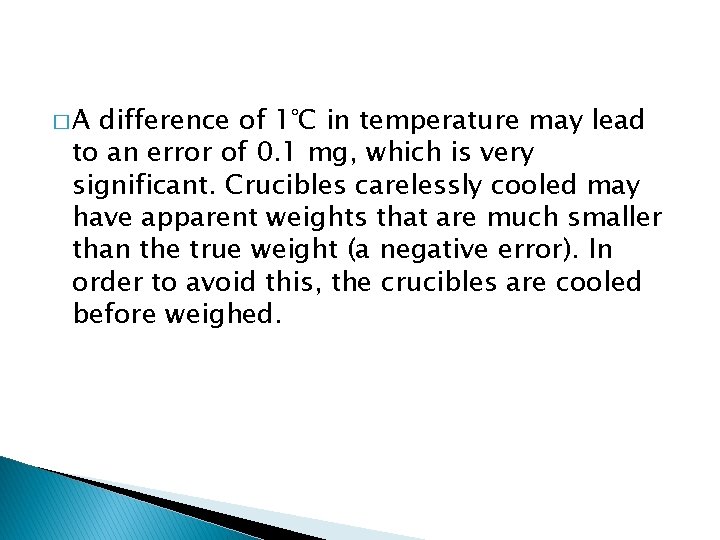
�A difference of 1°C in temperature may lead to an error of 0. 1 mg, which is very significant. Crucibles carelessly cooled may have apparent weights that are much smaller than the true weight (a negative error). In order to avoid this, the crucibles are cooled before weighed.

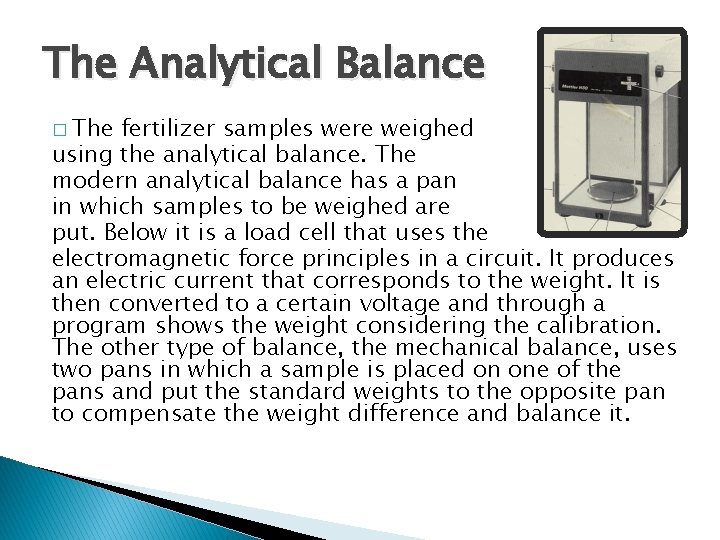
The Analytical Balance � The fertilizer samples were weighed using the analytical balance. The modern analytical balance has a pan in which samples to be weighed are put. Below it is a load cell that uses the electromagnetic force principles in a circuit. It produces an electric current that corresponds to the weight. It is then converted to a certain voltage and through a program shows the weight considering the calibration. The other type of balance, the mechanical balance, uses two pans in which a sample is placed on one of the pans and put the standard weights to the opposite pan to compensate the weight difference and balance it.

The Advantages of Weighing by Difference: � Weighing by difference takes into account only the lost weight while direct weighing takes into account the added weight. If you are doing direct weighing on an accurate balance even very fine dust particles will be added to the measured weight and thus will result to error, whereas when weighing by difference, the balance “zeros” out or tares out these additional weights. � It takes away the use of an intermediate object (e. g glassware) that may lead to an incomplete transfer or inaccurate results. � It avoids the transfer of material to an intermediate object (i. e. watch glass, filter paper) and therefore eliminates the possible loss by incomplete transfer to the final container.

Why Grind Fertilizer Samples? � The grinding fertilizer samples can help in the drying process. It makes the particles smaller and have a larger surface area which can evaporate more moisture than when it is clumped together (which makes it more difficult for the moisture to get out). � Occluded water that may be present in the samples may not be removed.

When is the weight of the sample considered to be constant? � When the difference between two successive weighings is equal to zero, the weight is considered constant, meaning the non essential water has been completely removed. But a difference of 0. 0002 to 0. 0003 grams between the previous weight and the newly acquired weight also signifies a constant weight because this range of values is valid for a macro scale analysis, since it is pointless to use a value more accurate than the accuracy attainable with the analytical method, as dictated by statistics.

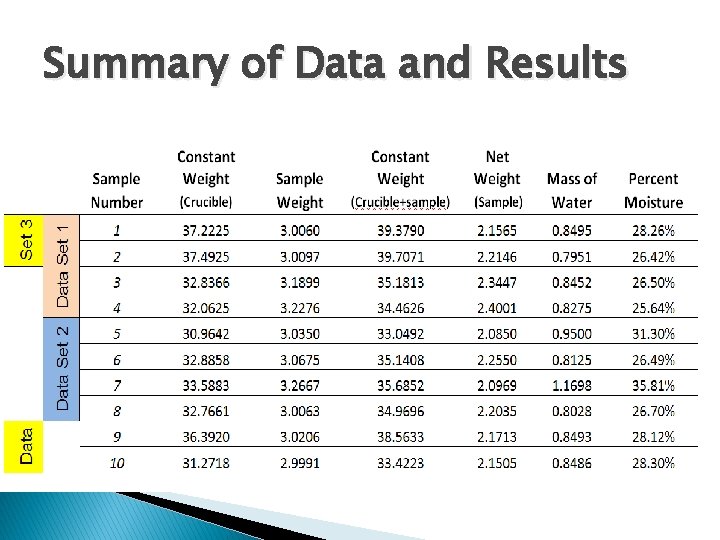
Summary of Data and Results

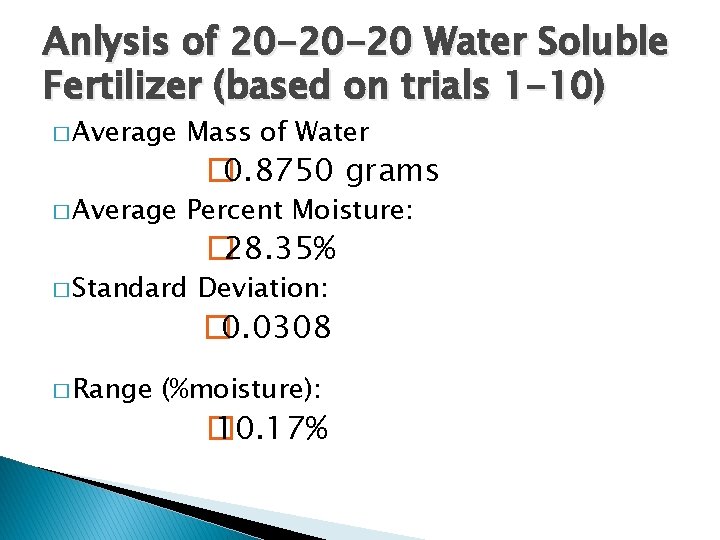
Anlysis of 20 -20 -20 Water Soluble Fertilizer (based on trials 1 -10) � Average Mass of Water � Average Percent Moisture: � Standard � Range � 0. 8750 grams � 28. 35% Deviation: � 0. 0308 (%moisture): � 10. 17%

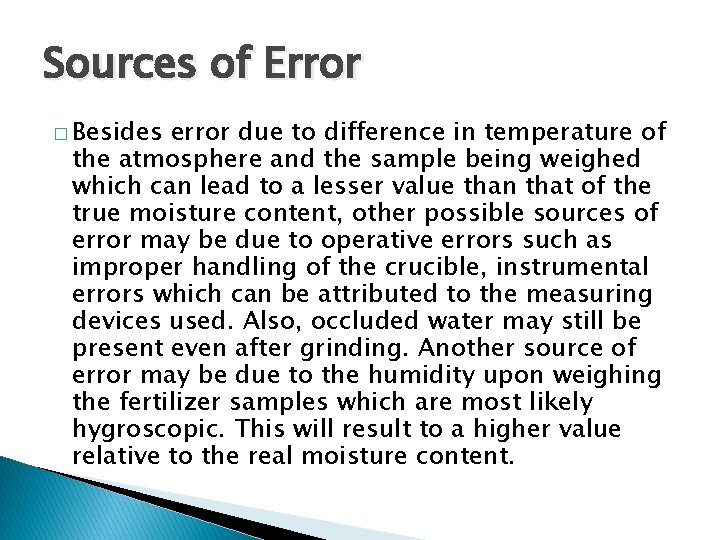
Sources of Error � Besides error due to difference in temperature of the atmosphere and the sample being weighed which can lead to a lesser value than that of the true moisture content, other possible sources of error may be due to operative errors such as improper handling of the crucible, instrumental errors which can be attributed to the measuring devices used. Also, occluded water may still be present even after grinding. Another source of error may be due to the humidity upon weighing the fertilizer samples which are most likely hygroscopic. This will result to a higher value relative to the real moisture content.
![Sources 1 Day R A Sr and Underwood A L Sources � � � [1] Day R. A. Sr. and Underwood A. L. ,](https://slidetodoc.com/presentation_image_h2/135fd75e1d26ab2902dc22c87def1fb5/image-21.jpg)
Sources � � � [1] Day R. A. Sr. and Underwood A. L. , Quantitative Analysis. Apson Enterprises: Quezon City, 1974. [2] Hamilton L. F. , Quantitative Chemical Analysis. New York: Mac. Millan, 1958. [3] Kolthoff, I. M. , and Sandell, E. B. , Textbook of Quantitative Inorganic Analysis. Macmillan: New York, 1949. [4] Kodama, K. Methods of Quantitative Inorganic Analysis: An Encyclopedia Of Gravimetric, Titrimetric And Colometric Methods. Interscience Publishers: New York, 1963. [5] Laitinen, H. A. and Harris, W. E. , Chemical Analysis: An advanced Text and Reference, 2 nd ed. Mc. Graw-Hill Book Co Inc. : New York, 1975. [5] Skoog, D. A. , West, D. M. , and Holler F. J. , Fundamentals of Analytical Chemistry, 8 th Ed. Brooks/Cole a Division of Thomson Learning Inc: United States of America, 2004.