Experiment 13 Urine Metabolic Screening Test Introduction Inborn

Experiment 13 Urine Metabolic Screening Test

Introduction Inborn Errors of Metabolism disorders characterized by disruption of enzymatic function in a metabolic pathway
Causes Ø Failure to inherit the gene to produce a particular enzyme • Inability of a reaction to occur at a sufficient rate • Metabolite accumulates in increased amounts • Reaction products fail to form • Reaction do not take place Ø Defect in transport system which control the passage of molecular across the membrane • Defects of absence of enzymes causing metabolic blocks

Clinical Manifestation Ø Mental retardation Ø Development delays Ø Seizures Ø Unexplained metabolic acidosis Ø Jaundice Ø Vomiting Ø Hepatomegaly Ø Abnormal facial and body features Ø Death
Urine Metabolic Screening Test Ø battery of tests that is performed on urine specimens to detect the possibility of a metabolic disorder Ø not specific and are used only as screening tests

the kidneys remove waste material, minerals, fluids, and other substances from the blood for elimination in the urine can contain hundreds of different bodily waste products diet, fluid intake, exercise, and kidney function, affect what is in the urine

Urine Ø good specimen of choice for the detection of most metabolic disturbances • accumulated metabolites “spill over”

Routine Urinalysis

Introduction Urine is formed from our kidneys and is an ultrafiltrate of plasma Our normal daily urine output is 1200 - 1500 ml, while a range of 600 – 2000 ml may still be considered normal Urine composition is of urea and other organic chemicals dissolved in water
Objectives To note the physical characteristics of the urine sample To perform a routine chemical examination on the urine sample (using a reagent strip) To identify materials present in the urine through microscopic analysis To be able to correlate physical, chemical and microscopic observations and know their clinical significances
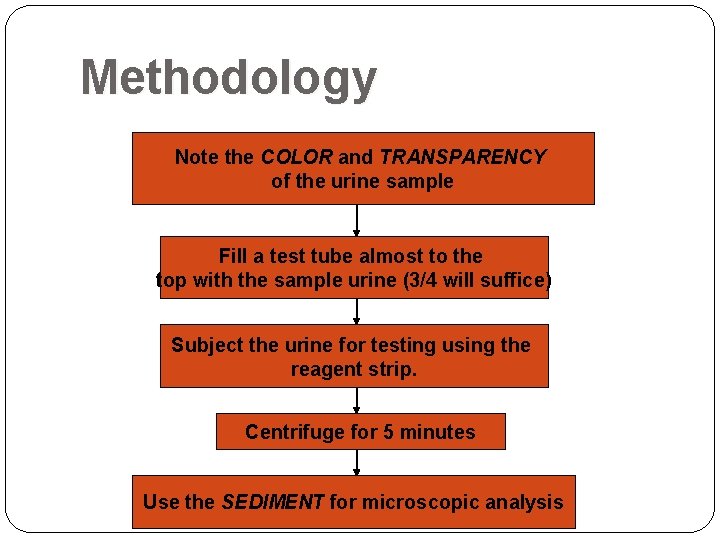
Methodology Note the COLOR and TRANSPARENCY of the urine sample Fill a test tube almost to the top with the sample urine (3/4 will suffice) Subject the urine for testing using the reagent strip. Centrifuge for 5 minutes Use the SEDIMENT for microscopic analysis
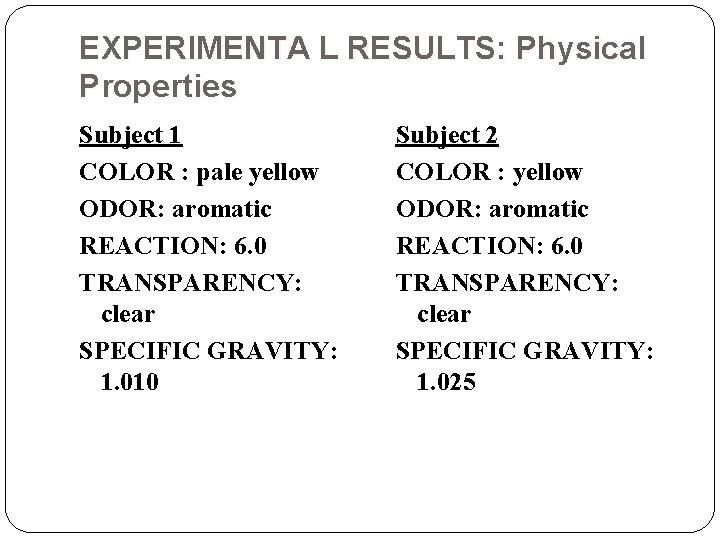
EXPERIMENTA L RESULTS: Physical Properties Subject 1 COLOR : pale yellow ODOR: aromatic REACTION: 6. 0 TRANSPARENCY: clear SPECIFIC GRAVITY: 1. 010 Subject 2 COLOR : yellow ODOR: aromatic REACTION: 6. 0 TRANSPARENCY: clear SPECIFIC GRAVITY: 1. 025
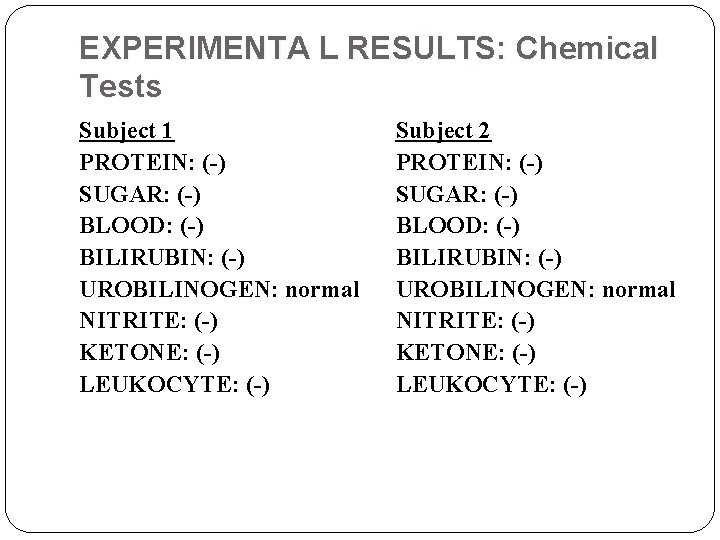
EXPERIMENTA L RESULTS: Chemical Tests Subject 1 PROTEIN: (-) SUGAR: (-) BLOOD: (-) BILIRUBIN: (-) UROBILINOGEN: normal NITRITE: (-) KETONE: (-) LEUKOCYTE: (-) Subject 2 PROTEIN: (-) SUGAR: (-) BLOOD: (-) BILIRUBIN: (-) UROBILINOGEN: normal NITRITE: (-) KETONE: (-) LEUKOCYTE: (-)
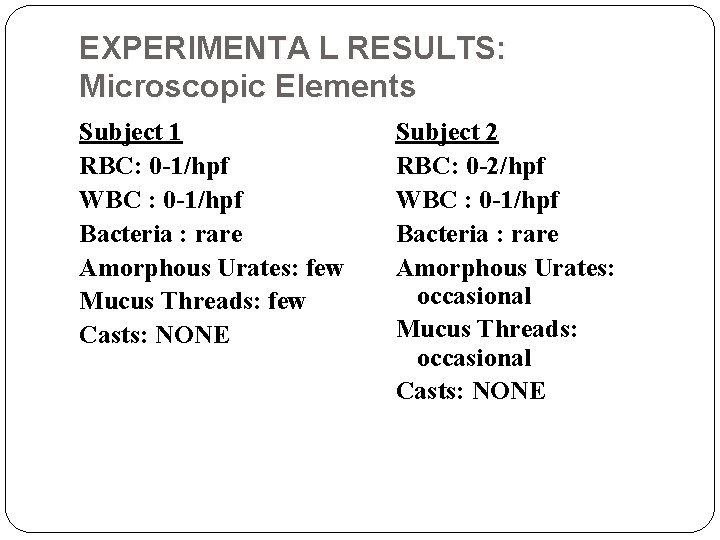
EXPERIMENTA L RESULTS: Microscopic Elements Subject 1 RBC: 0 -1/hpf WBC : 0 -1/hpf Bacteria : rare Amorphous Urates: few Mucus Threads: few Casts: NONE Subject 2 RBC: 0 -2/hpf WBC : 0 -1/hpf Bacteria : rare Amorphous Urates: occasional Mucus Threads: occasional Casts: NONE
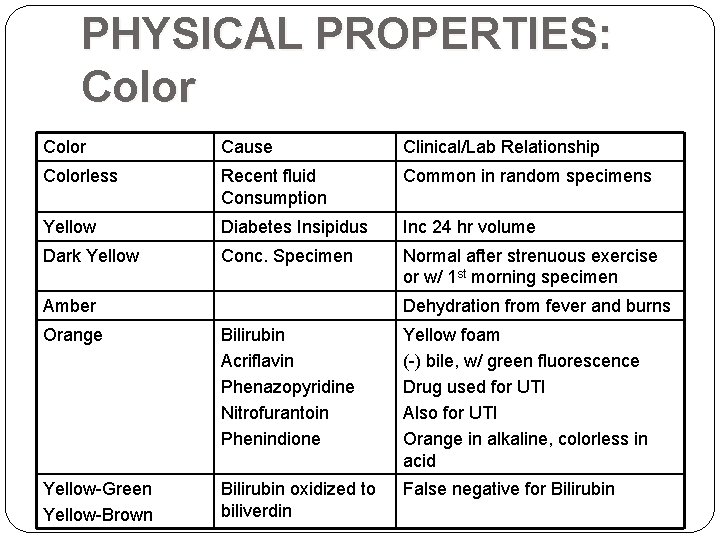
PHYSICAL PROPERTIES: Color Cause Clinical/Lab Relationship Colorless Recent fluid Consumption Common in random specimens Yellow Diabetes Insipidus Inc 24 hr volume Dark Yellow Conc. Specimen Normal after strenuous exercise or w/ 1 st morning specimen Amber Dehydration from fever and burns Orange Bilirubin Acriflavin Phenazopyridine Nitrofurantoin Phenindione Yellow foam (-) bile, w/ green fluorescence Drug used for UTI Also for UTI Orange in alkaline, colorless in acid Yellow-Green Yellow-Brown Bilirubin oxidized to biliverdin False negative for Bilirubin
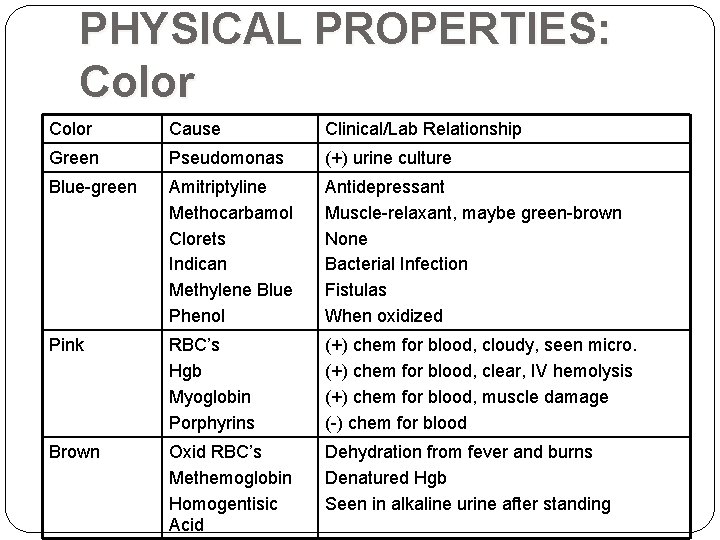
PHYSICAL PROPERTIES: Color Cause Clinical/Lab Relationship Green Pseudomonas (+) urine culture Blue-green Amitriptyline Methocarbamol Clorets Indican Methylene Blue Phenol Antidepressant Muscle-relaxant, maybe green-brown None Bacterial Infection Fistulas When oxidized Pink RBC’s Hgb Myoglobin Porphyrins (+) chem for blood, cloudy, seen micro. (+) chem for blood, clear, IV hemolysis (+) chem for blood, muscle damage (-) chem for blood Brown Oxid RBC’s Methemoglobin Homogentisic Acid Dehydration from fever and burns Denatured Hgb Seen in alkaline urine after standing
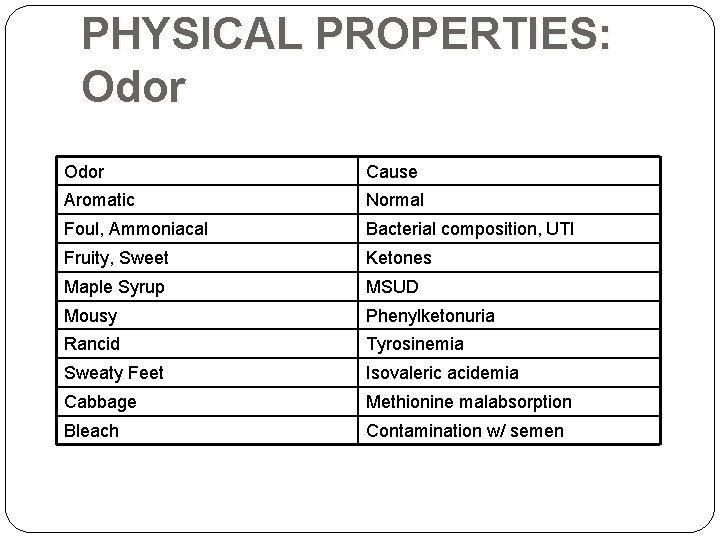
PHYSICAL PROPERTIES: Odor Cause Aromatic Normal Foul, Ammoniacal Bacterial composition, UTI Fruity, Sweet Ketones Maple Syrup MSUD Mousy Phenylketonuria Rancid Tyrosinemia Sweaty Feet Isovaleric acidemia Cabbage Methionine malabsorption Bleach Contamination w/ semen
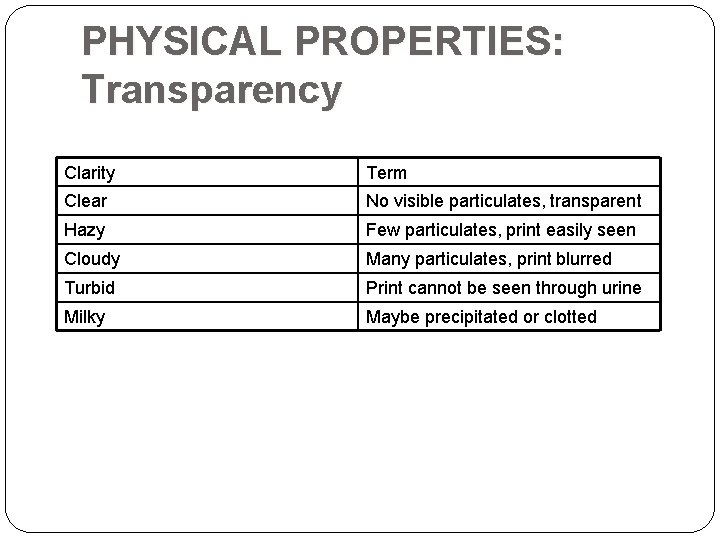
PHYSICAL PROPERTIES: Transparency Clarity Term Clear No visible particulates, transparent Hazy Few particulates, print easily seen Cloudy Many particulates, print blurred Turbid Print cannot be seen through urine Milky Maybe precipitated or clotted

Chemical Examination p. H: can range from 4. 5 -8. 0 Specific Gravitiy: 1. 015 -1. 025 Protein: negative or trace Sugar: negative or trace Blood: negative Bilirubin: negative Urobilinogen: negative Nitrite: negative Ketone: negative Leukocyte: negative
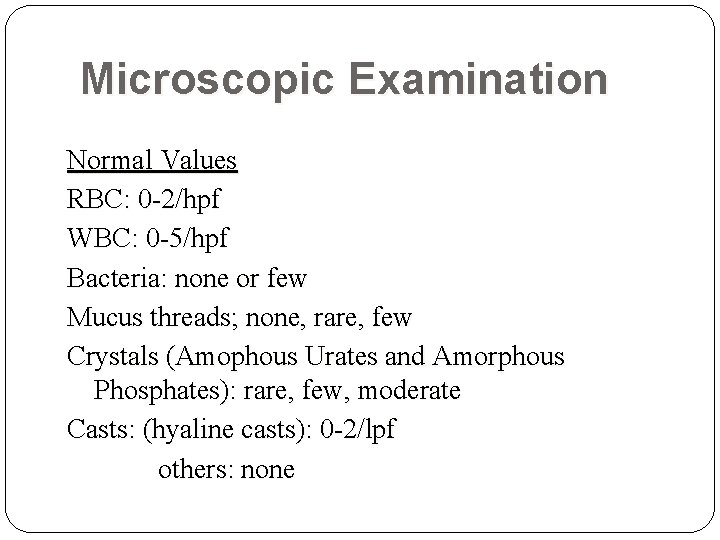
Microscopic Examination Normal Values RBC: 0 -2/hpf WBC: 0 -5/hpf Bacteria: none or few Mucus threads; none, rare, few Crystals (Amophous Urates and Amorphous Phosphates): rare, few, moderate Casts: (hyaline casts): 0 -2/lpf others: none

Ferric Chloride Test Screening Tests for Certain Inborn Errors of Metabolism
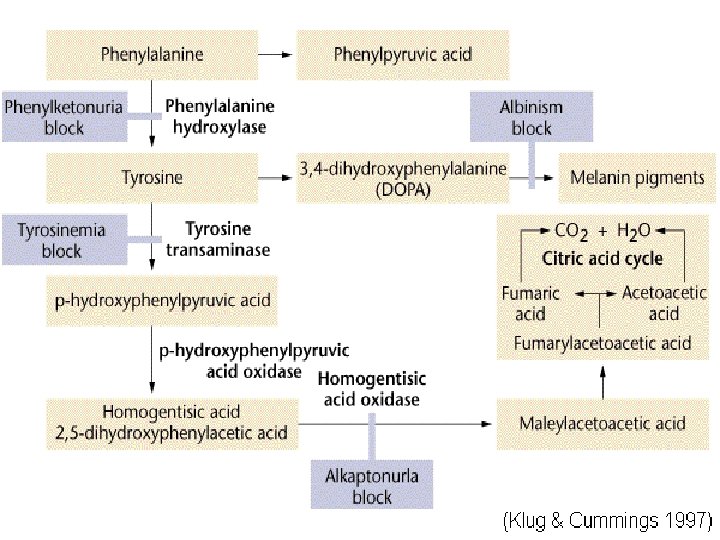
Ferric Chloride Tests for the presence of high levels of phenylpyruvate in urine (phenylketonuria) Detects compounds such as aromatic hydroxyl groups, phenols and enols Transient or permanent coloration (usually purple, green or blue) indicates the presence of aromatic hydroxyl compounds, phenols and enols
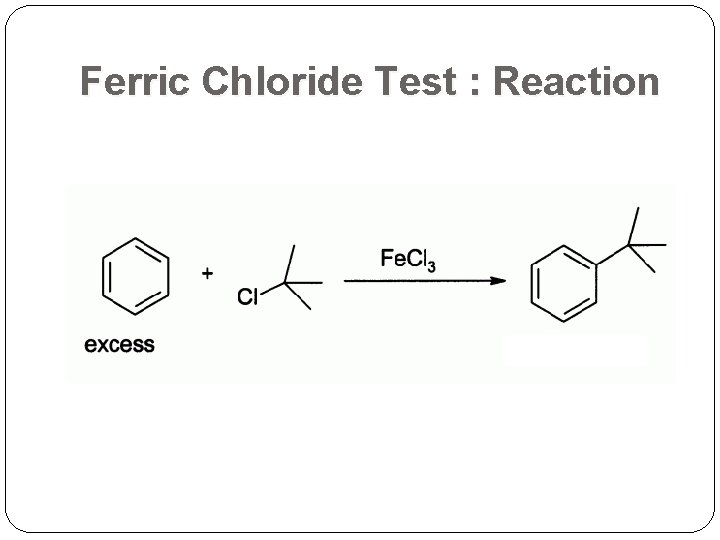
Ferric Chloride Test : Reaction

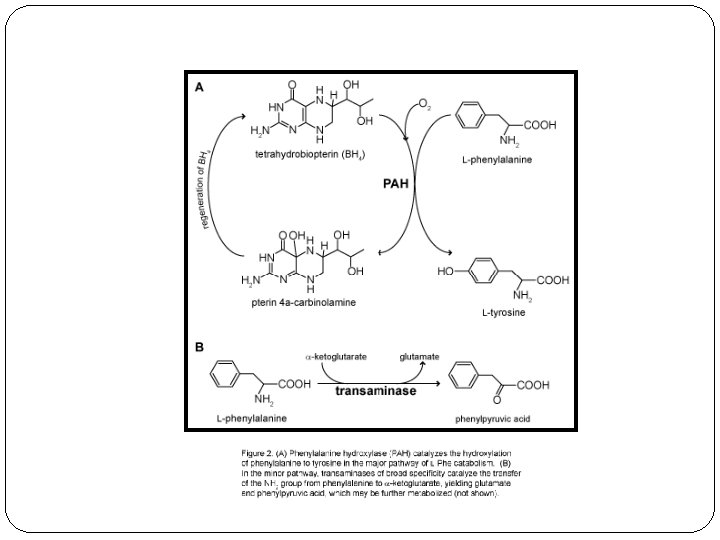
Ferric Chloride Test The OH (hydroxy group) which is attached directly to an aromatic nucleus (e. g. Benzene)is detected by the Ferric chloride Phenols will typically yield dramatic purple, blue, red or green color as an indication of a positive test. Aromatic acids will test as a beige-tan color. (+) Aliphatic acids non-aromatic organic acids (Acetic acid)

Ferric Chloride Test Ferric ion forms a colored complex with phenylpyruvate: blue green color blue-green color indicates the presence of metabolites of phenylalanine ketone phenylpyruvic acid in the urine gives the disease its name & a green color in the ferric chloride test
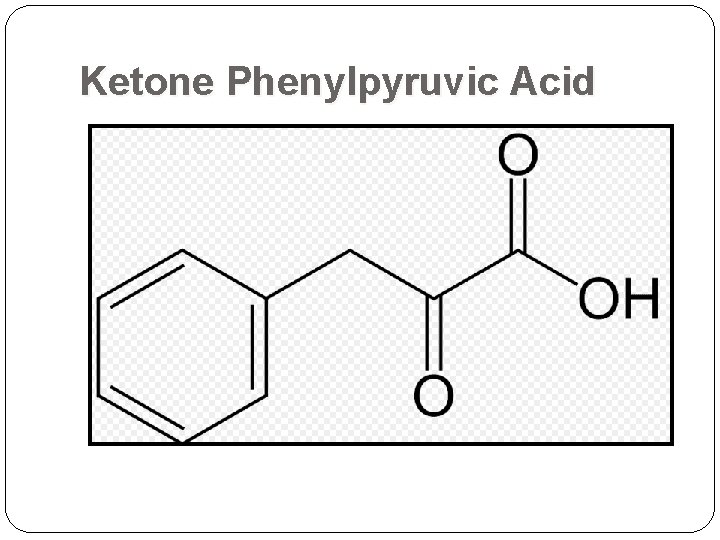
Ketone Phenylpyruvic Acid
Ferric Chloride Test Why is it no longer used? It is complexed with other compounds, producing interfering colors May mask the blue-green color of the ferric complex with phenylpyruvate Relatively high concentrations of phenylpyruvate must be present in order for there to be appreciate development of the characteristic color change assay is less sensitive, and more prone to misinterpretation

Phenylketonuria Inherited disorder (autosomal recessive) of phenylalanine metabolism causing severe mental retardation Diagnosed by finding excess phenylpyruvic acid in serum and urine Lack of enzyme phenylalanine hydroxylase causes accumulation of phenylalanine in plasma Urine: “Mousy odor” Physical: Eczema Fair coloring as a result of tyrosine deficiency(pigmentation metabolite, melanin) Enzyme: Phenylalanine Hydroxylase the enzyme locus is on chromosome arm 12 q
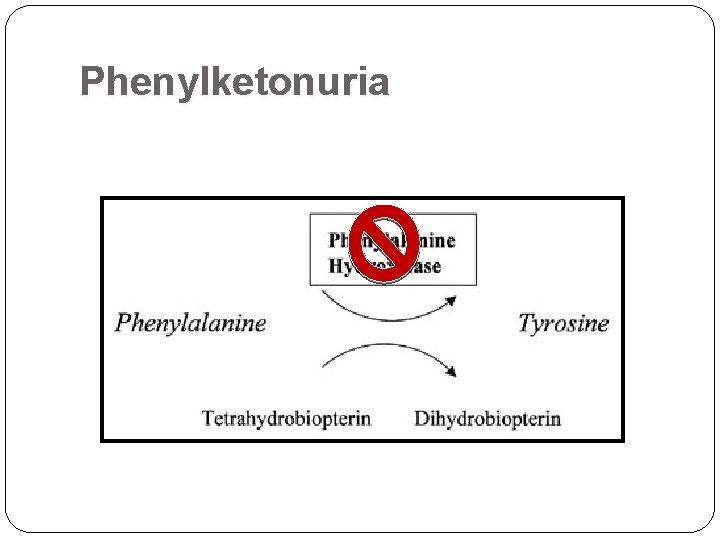
Phenylketonuria
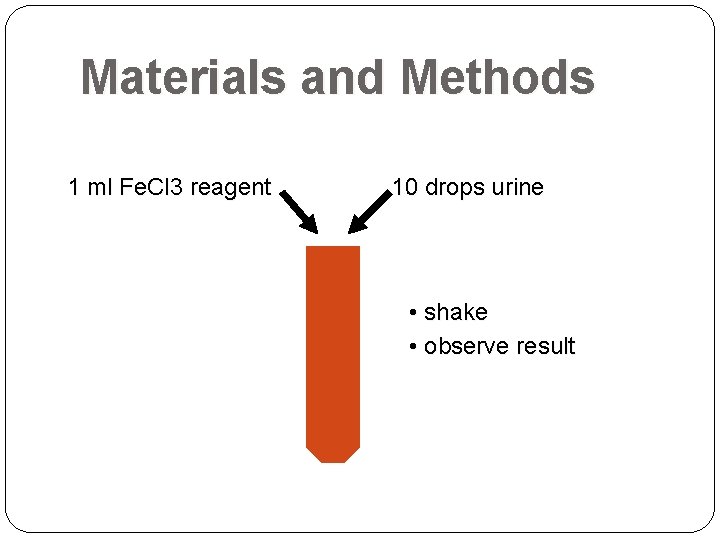
Materials and Methods 1 ml Fe. Cl 3 reagent 10 drops urine • shake • observe result
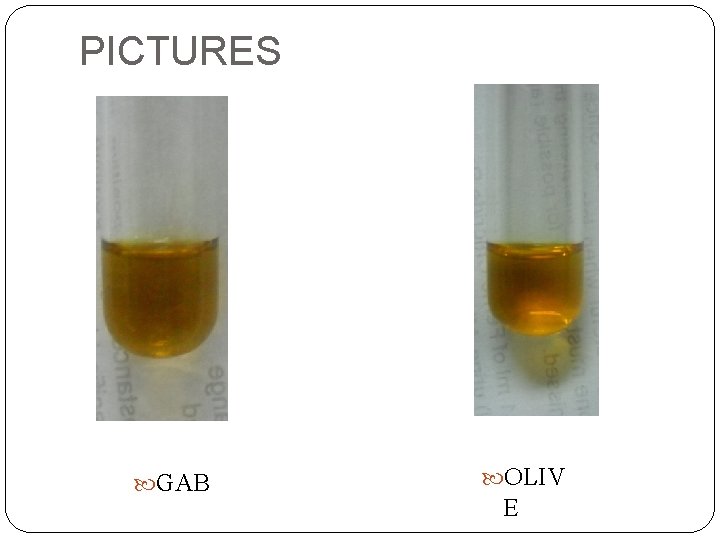
PICTURES GAB OLIV E
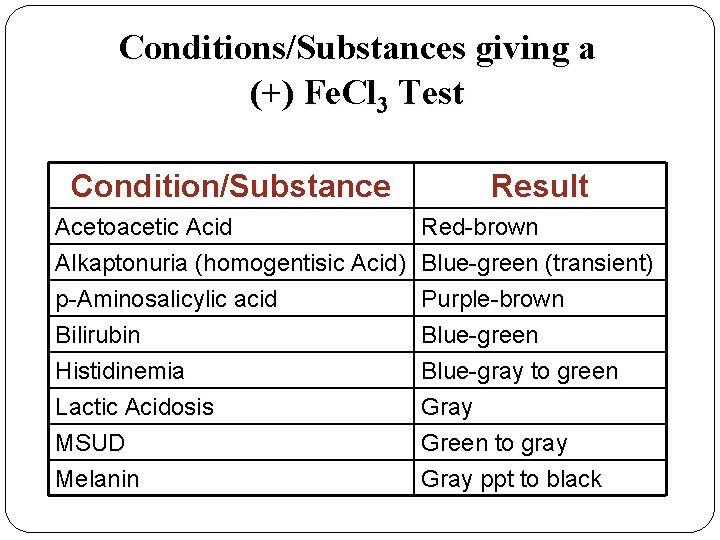
Conditions/Substances giving a (+) Fe. Cl 3 Test Condition/Substance Result Acetoacetic Acid Red-brown Alkaptonuria (homogentisic Acid) Blue-green (transient) p-Aminosalicylic acid Purple-brown Bilirubin Blue-green Histidinemia Blue-gray to green Lactic Acidosis Gray MSUD Green to gray Melanin Gray ppt to black
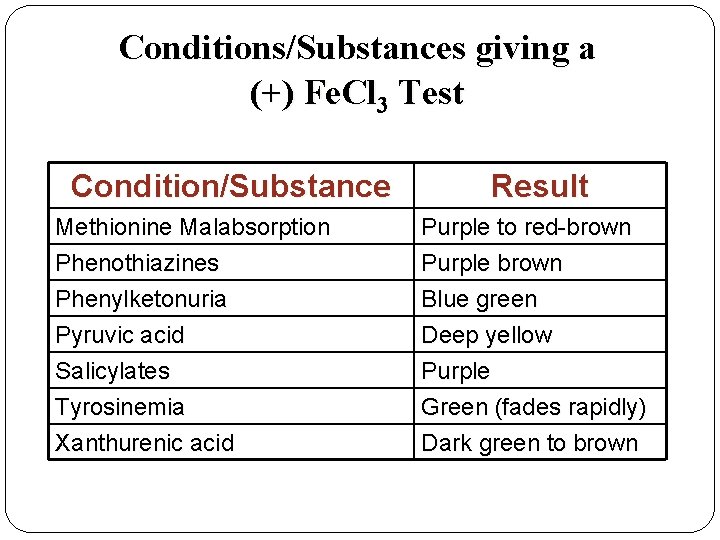
Conditions/Substances giving a (+) Fe. Cl 3 Test Condition/Substance Result Methionine Malabsorption Phenothiazines Purple to red-brown Purple brown Phenylketonuria Pyruvic acid Salicylates Tyrosinemia Xanthurenic acid Blue green Deep yellow Purple Green (fades rapidly) Dark green to brown

Experimental Results Both urine samples (GAB&OLIVE): NEGATIVE

Benedict’s Test Screening Tests for Certain Inborn Errors of Metabolism
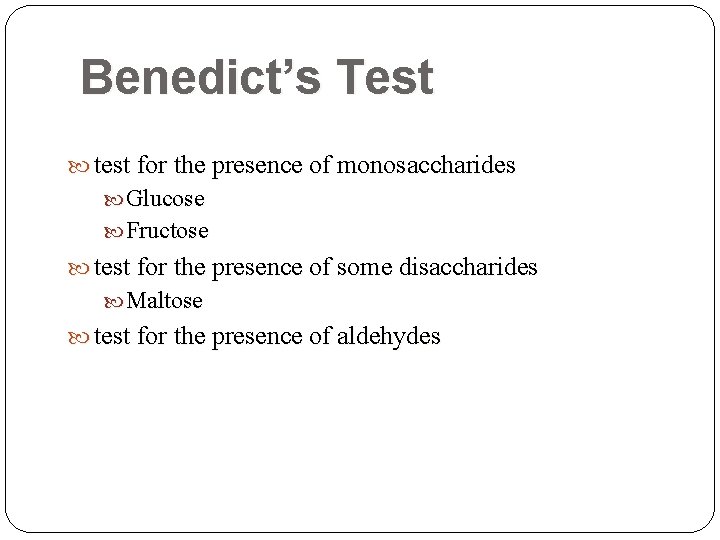
Benedict’s Test test for the presence of monosaccharides Glucose Fructose test for the presence of some disaccharides Maltose test for the presence of aldehydes

Benedict’s Test Benedict’s reagent can be used to test for presence of glucose in urine Indication of diabetes Heating a Benedict’s solution mixed with monosaccharides will produce a reddish-orange color
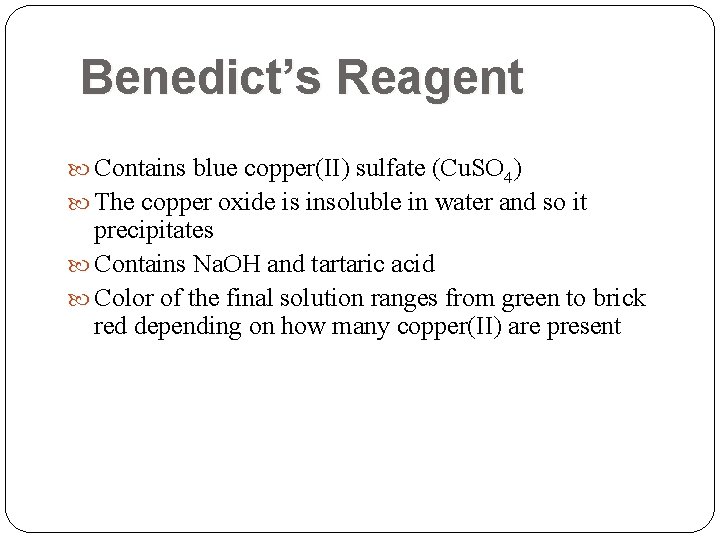
Benedict’s Reagent Contains blue copper(II) sulfate (Cu. SO 4) The copper oxide is insoluble in water and so it precipitates Contains Na. OH and tartaric acid Color of the final solution ranges from green to brick red depending on how many copper(II) are present
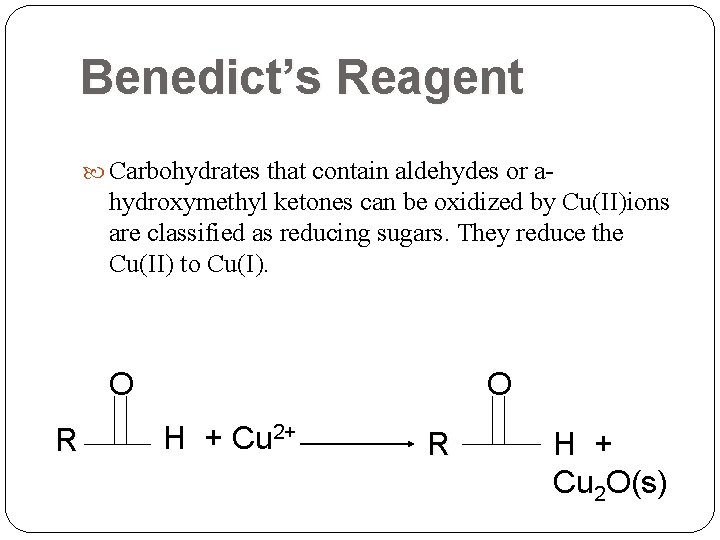
Benedict’s Reagent Carbohydrates that contain aldehydes or a- hydroxymethyl ketones can be oxidized by Cu(II)ions are classified as reducing sugars. They reduce the Cu(II) to Cu(I). O R O H + Cu 2+ R H + Cu 2 O(s)
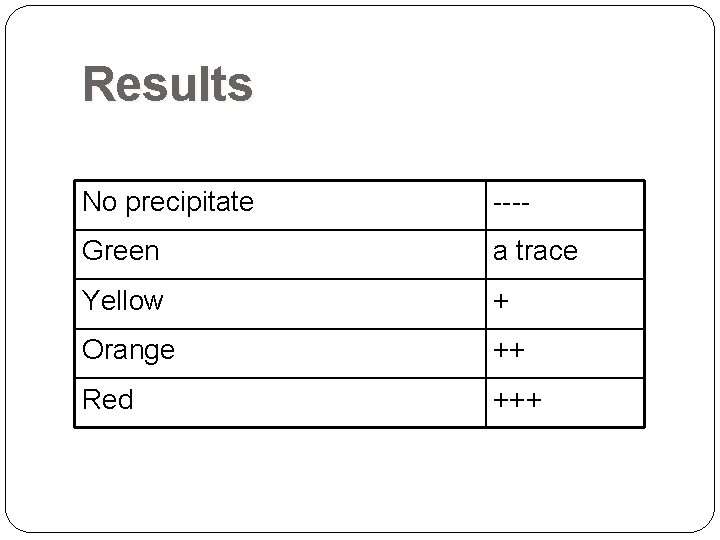
Results No precipitate ---- Green a trace Yellow + Orange ++ Red +++
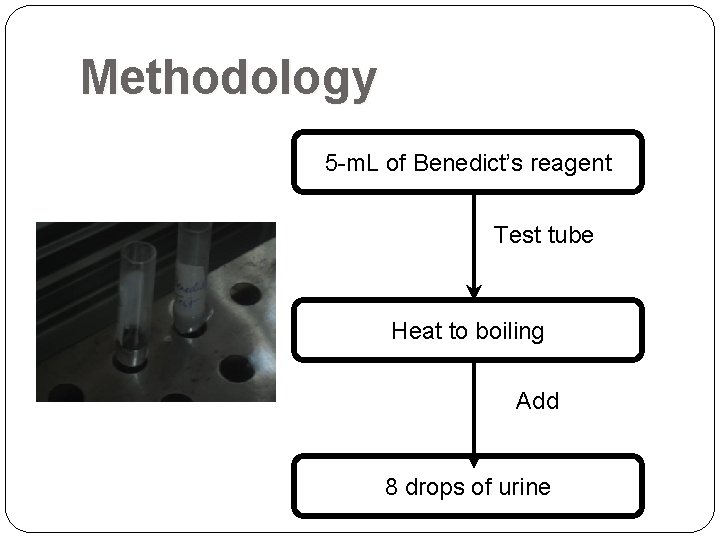
Methodology 5 -m. L of Benedict’s reagent Test tube Heat to boiling Add 8 drops of urine
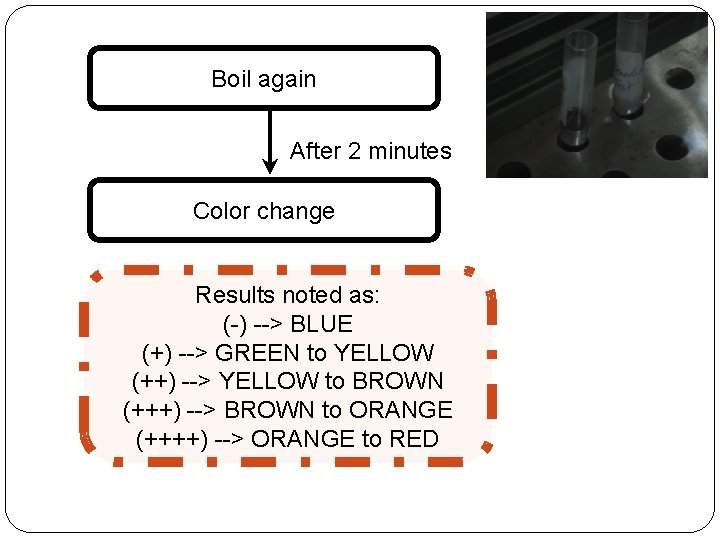
Boil again After 2 minutes Color change Results noted as: (-) --> BLUE (+) --> GREEN to YELLOW (++) --> YELLOW to BROWN (+++) --> BROWN to ORANGE (++++) --> ORANGE to RED
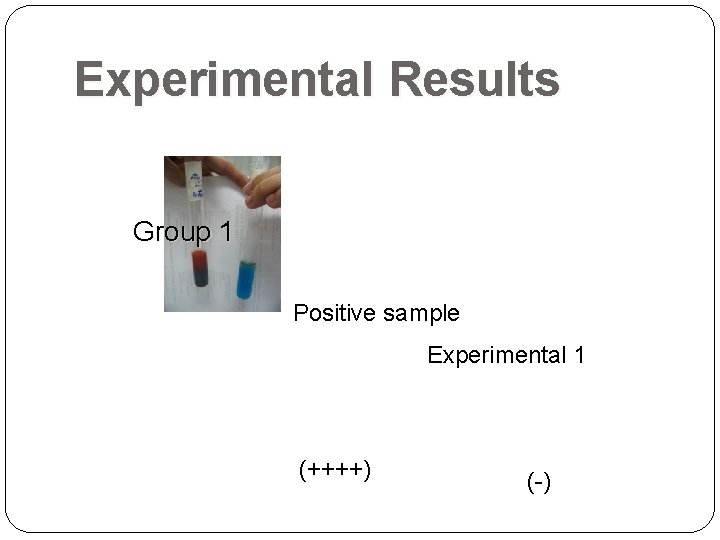
Experimental Results Group 1 Positive sample Experimental 1 (++++) (-)

Group 2 Positive sample (++++) Experimental 2 (+)
Benedict’s Test detect the presence of reducing sugars Reducing sugars - sugars with a free aldehyde or ketone group Aldehyde groups are oxidized to carboxylic acids Ketoses can also be reducing sugars because they can isomerize (tautomerisation) to aldoses via an enediol
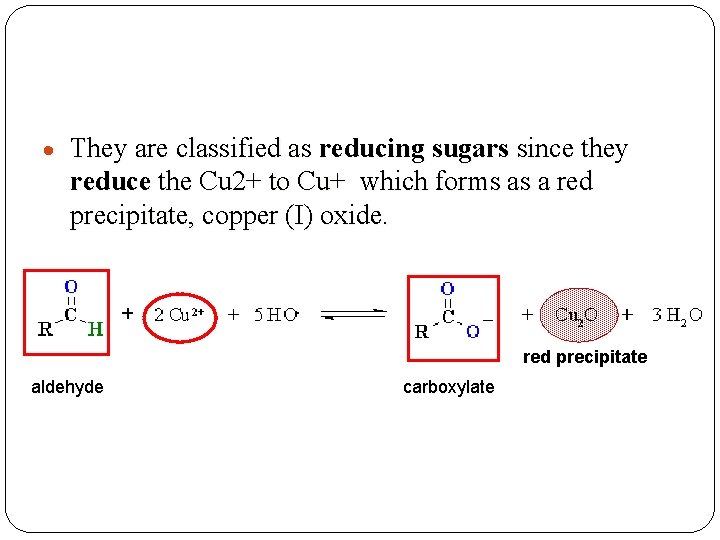
They are classified as reducing sugars since they reduce the Cu 2+ to Cu+ which forms as a red precipitate, copper (I) oxide. red precipitate aldehyde carboxylate
Benedict’s Reagent Deep-blue alkaline solution of copper sulfate, sodium hydroxide, and tartaric acid The copper sulfate (Cu. SO 4) reacts with electrons from the aldehyde or ketone group of the reducing sugar to form cuprous oxide (Cu 2 O), a red-brown precipitate. 2 Cu++ Cu. SO 4 Cu++ + SO 4 -+ Reducing Sugar Cu+ (electron donor) Cu+ Cu 2 O (precipitate) The final color - how much of this precipitate was formed gives an indication of how much reducing sugar was present. Increasing amounts of reducing sugar bluegreenyellow brownorangered
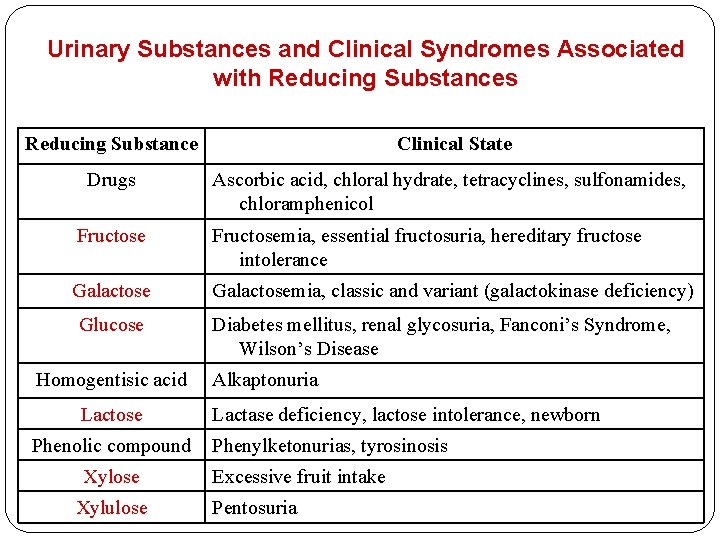
Urinary Substances and Clinical Syndromes Associated with Reducing Substances Reducing Substance Clinical State Drugs Ascorbic acid, chloral hydrate, tetracyclines, sulfonamides, chloramphenicol Fructosemia, essential fructosuria, hereditary fructose intolerance Galactosemia, classic and variant (galactokinase deficiency) Glucose Homogentisic acid Lactose Phenolic compound Xylose Xylulose Diabetes mellitus, renal glycosuria, Fanconi’s Syndrome, Wilson’s Disease Alkaptonuria Lactase deficiency, lactose intolerance, newborn Phenylketonurias, tyrosinosis Excessive fruit intake Pentosuria
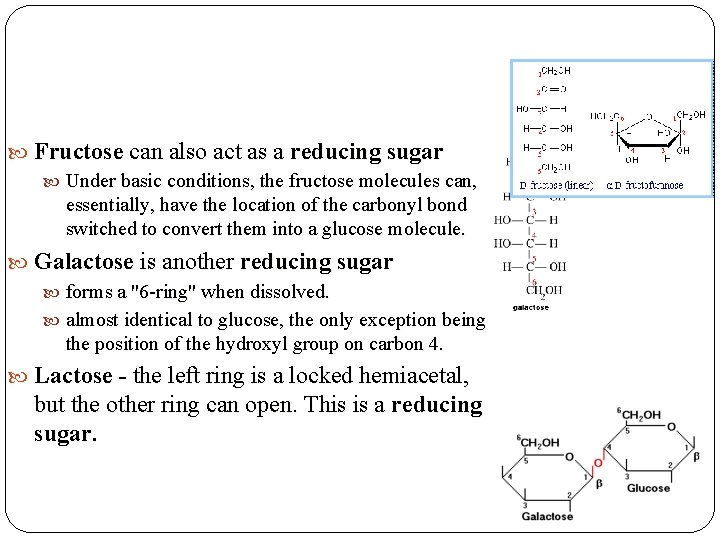
Fructose can also act as a reducing sugar Under basic conditions, the fructose molecules can, essentially, have the location of the carbonyl bond switched to convert them into a glucose molecule. Galactose is another reducing sugar forms a "6 -ring" when dissolved. almost identical to glucose, the only exception being the position of the hydroxyl group on carbon 4. Lactose - the left ring is a locked hemiacetal, but the other ring can open. This is a reducing sugar.
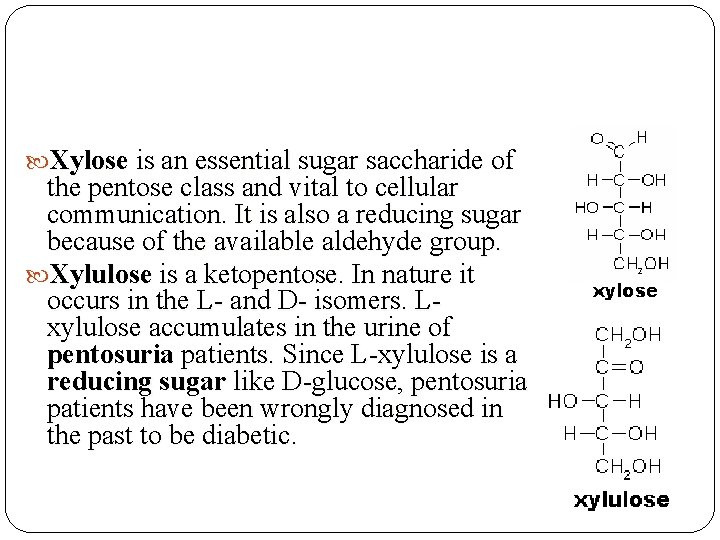
Xylose is an essential sugar saccharide of the pentose class and vital to cellular communication. It is also a reducing sugar because of the available aldehyde group. Xylulose is a ketopentose. In nature it occurs in the L- and D- isomers. Lxylulose accumulates in the urine of pentosuria patients. Since L-xylulose is a reducing sugar like D-glucose, pentosuria patients have been wrongly diagnosed in the past to be diabetic.

Nitrosonaphthol Test Screening Tests for Certain Inborn Errors of Metabolism
Nitrosonaphtol Test Screening for inborn error of TYROSINE metabolism Tyrosine
Nitrosonaphtol Reagent 1 -nitroso-2 -naphthol organic compound yellow-brown, crystalline melting point: 107°C commonly used to determine cobalt moderately hazardous reacts with substituted phenolic compounds
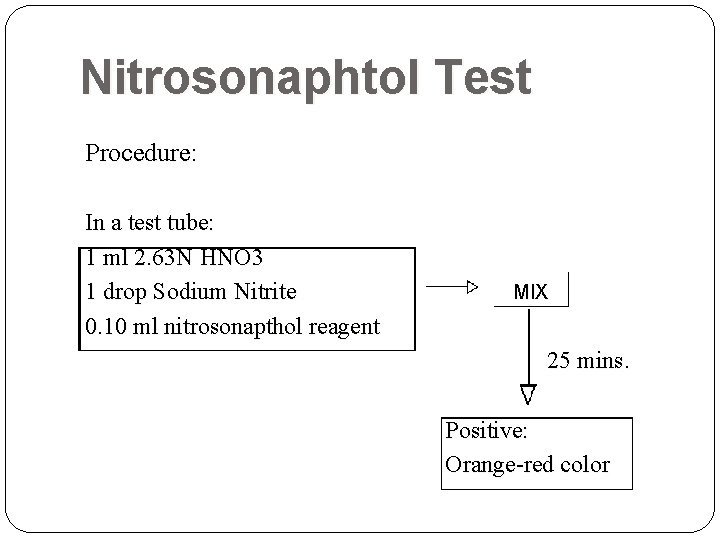
Nitrosonaphtol Test Procedure: In a test tube: 1 ml 2. 63 N HNO 3 1 drop Sodium Nitrite 0. 10 ml nitrosonapthol reagent MIX 25 mins. Positive: Orange-red color
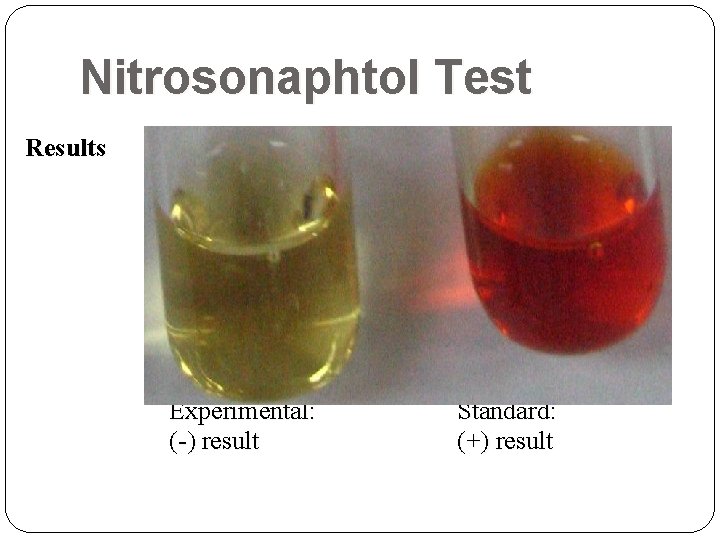
Nitrosonaphtol Test Results Experimental: (-) result Standard: (+) result

Discussion Mechanism 1 -nitroso-2 -naphthol reacts with tyrosine or its metabolites to produce a positive result. Reacts with: Tyrosine and its metabolites 5 -hydroxyindoles Substituted guiacols

Nitrosonaphtol Test False Positive Result IV fluids containing homovanillic acid 5 -HIAA N-acetyltyrosine

Tyrosinosis Very rare hereditary disorder of Tyrosine metabolism Defective formation of p-hydroxyphenylpyruvic acid oxidase or of tyrosine transaminase Enhanced urinary excretion of phydroxyphenylpyruvic acid and other tyrosyl metabolites

Nitroprusside Test Screening Tests for Certain Inborn Errors of Metabolism
Introduction Alternative Names: Acetone bodies; Ketones - serum; Ketone bodies – serum Measures the amount of ketones in the blood. Any amount of detectable ketones is considered abnormal. Used in the screening of cystinuria, homocystinuria and β-mercaptolactate cysteine disulfiduria
Cystinuria Renal transport defect Urinary excretion of cystine is markedly increase Complication: formation of cystine stones Homosytinuria Defect in cystathione synthase Elevated levels of homocystinuria in blood and urine
A person may have these disorders if he tests positive
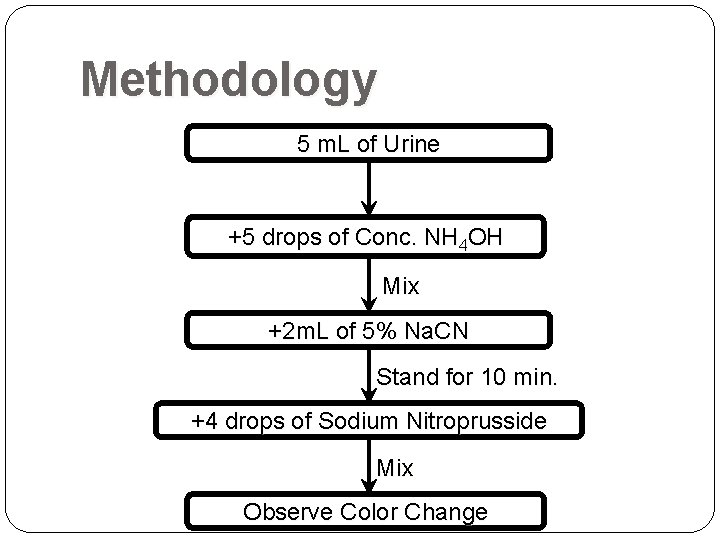
Methodology 5 m. L of Urine +5 drops of Conc. NH 4 OH Mix +2 m. L of 5% Na. CN Stand for 10 min. +4 drops of Sodium Nitroprusside Mix Observe Color Change

Experimental Results The solution produced was yellow. Hence, the subject tested negative for the test.
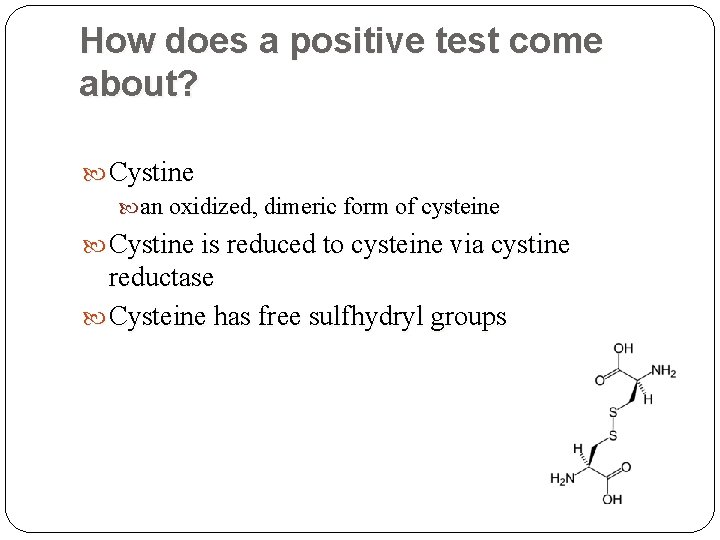
How does a positive test come about? Cystine an oxidized, dimeric form of cysteine Cystine is reduced to cysteine via cystine reductase Cysteine has free sulfhydryl groups

In the Nitroprusside Reaction, the sulhydryl groups react with nitroprusside pink color Cystine crystals are hexagonal and colorless. They become pink in the nitroprusside reaction.

Positive Test Result + - pink ++ - pinkish +++ - purple ++++ - dark purple

Cetyltrimethylammonium Bromide Test (CAB) Screening Tests for Certain Inborn Errors of Metabolism
Cetyltrimethyammonium Bromide Test Turbidometric technique Uses quaternary ammonium compounds e. g. CAB Used for both qualitative and quantitative determination of urinary mucopolysaccharides and glycosaminoglycans in various forms of mucopolysaccharidoses
Mucopolysaccharidoses (MPS) Constitute a large and heterogeneous subgroup among the lysosomal storage diseases (LSD) Caused by deficiency of specific enzymes, which are responsible for glycosaminoglycan (GAG) breakdown during different steps of its degradation pathway Differential diagnosis is important for a correct prognosis, definition of management strategies, genetic counseling, prenatal diagnosis, and prediction of future cases in the family

Objective To determine the presence of urinary mucopolysaccharides and glycosaminoglycans in the urine
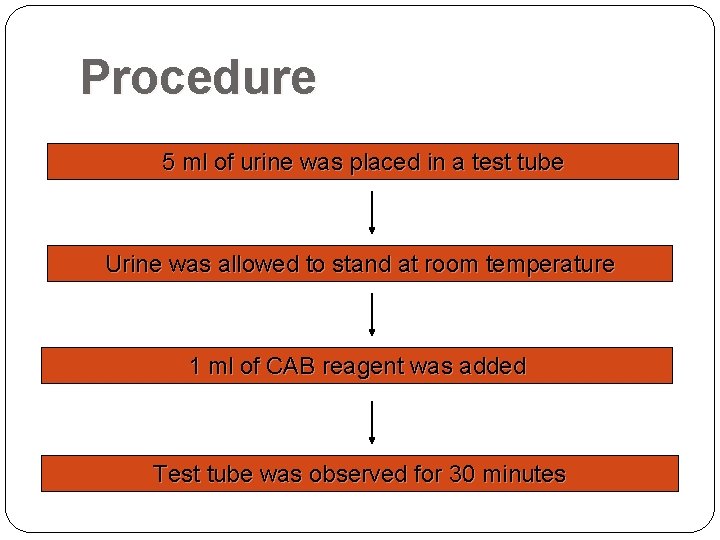
Procedure 5 ml of urine was placed in a test tube Urine was allowed to stand at room temperature 1 ml of CAB reagent was added Test tube was observed for 30 minutes

Results Team 1: no turbidity observed (0) Team 2: positive turbidity (1)
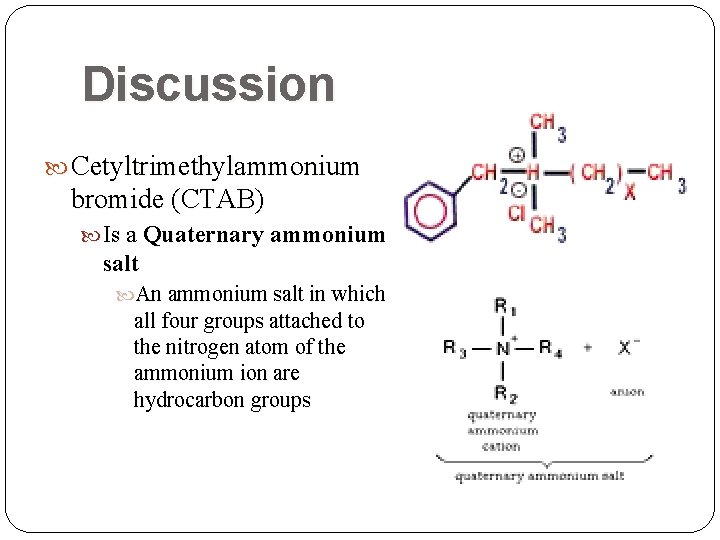
Discussion Cetyltrimethylammonium bromide (CTAB) Is a Quaternary ammonium salt An ammonium salt in which all four groups attached to the nitrogen atom of the ammonium ion are hydrocarbon groups
Quaternary ammonium compounds fix soluble acid mucopolysaccharides (e. g. , mucin) by forming highly insoluble complexes For example, an aqueous solution of heparin sodium salt is mixed with the quaternary ammonium salt Consequently, heparin in the heparin complex is a mucopolysaccharide chain with negative sulfate groups in it associated with the positive quaternary ammonium groups

Lysosomal storage disorders are variably referred to as simply storage disorders, lipidoses, mucopolysaccharidoses, or mucolipidoses Caused by deficiencies of enzymes which are involved in the degradation sequence of GAGs Diagnosis is through urine testing for the presence of increased amounts of GAGs
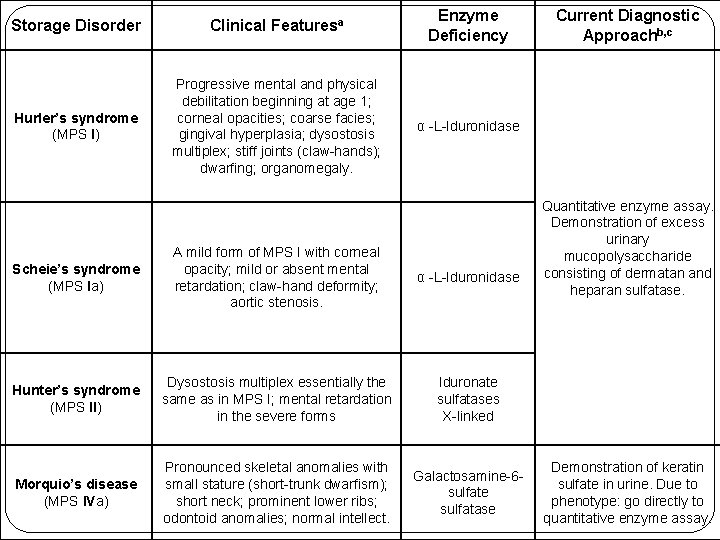
Storage Disorder Clinical Featuresa Enzyme Deficiency Hurler’s syndrome (MPS I) Progressive mental and physical debilitation beginning at age 1; corneal opacities; coarse facies; gingival hyperplasia; dysostosis multiplex; stiff joints (claw-hands); dwarfing; organomegaly. α -L-Iduronidase Scheie’s syndrome (MPS Ia) A mild form of MPS I with corneal opacity; mild or absent mental retardation; claw-hand deformity; aortic stenosis. α -L-Iduronidase Hunter’s syndrome (MPS II) Dysostosis multiplex essentially the same as in MPS I; mental retardation in the severe forms Iduronate sulfatases X-linked Morquio’s disease (MPS IVa) Pronounced skeletal anomalies with small stature (short-trunk dwarfism); short neck; prominent lower ribs; odontoid anomalies; normal intellect. Galactosamine-6 sulfate sulfatase Current Diagnostic Approachb, c Quantitative enzyme assay. Demonstration of excess urinary mucopolysaccharide consisting of dermatan and heparan sulfatase. Demonstration of keratin sulfate in urine. Due to phenotype: go directly to quantitative enzyme assay.

Ninhydrin Test Screening Tests for Certain Inborn Errors of Metabolism

Introduction Ninhydrin (Triketohydrindane hydrate) C 9 H 6 O 4 Appearance: white pale yellow crystals a chemical used to detect ammonia or primary and secondary amines produces a deep blue or purple coloration Ninhydrin Reagent Solution: Ninhydrin: 0. 35 g ethanol or acetone/butanol so-propanol: 100 ml
Introduction for detection and quantification of amino acids in substances for experiment: in urine can be used qualitatively (e. g. for chromatographic visualization) or quantitatively (e. g. for peptide sequencing) once used for fingerprint detection (forensics): amines leftover from peptides & proteins (terminal amines or lysine residues) sloughed off in fingerprints react with ninhydrin
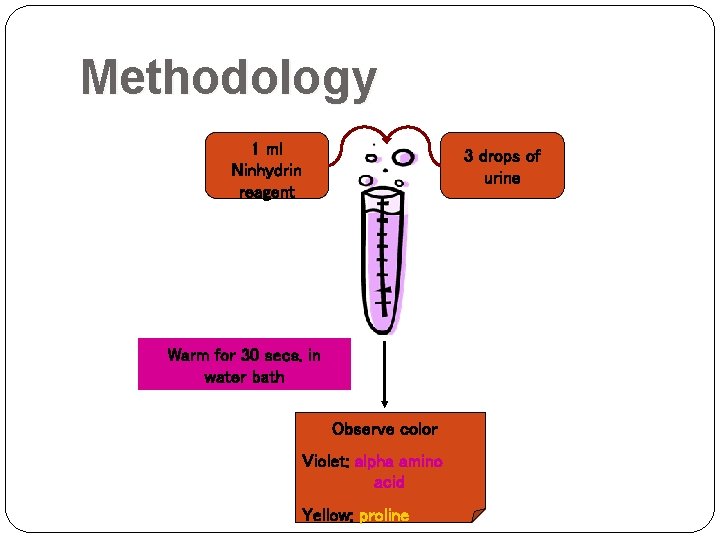
Methodology 1 ml Ninhydrin reagent 3 drops of urine Warm for 30 secs. in water bath Observe color Violet: alpha amino acid Yellow: proline

Experimental Results Group 1: (-) colorless Group 2: (+) slightly bluish coloration
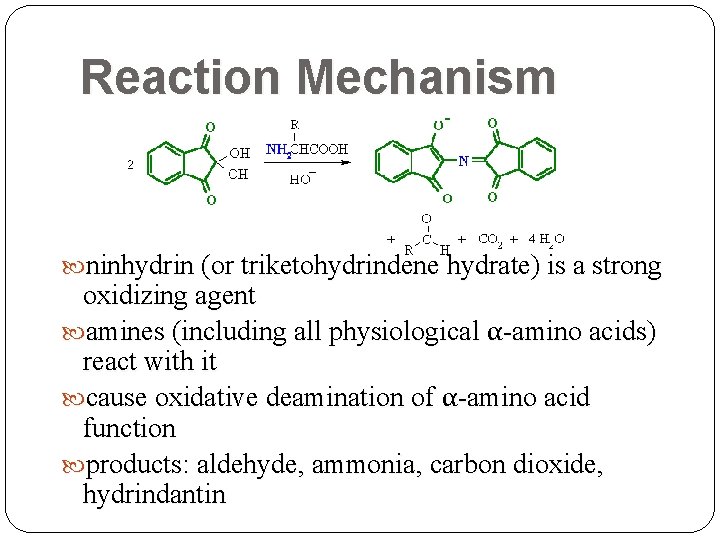
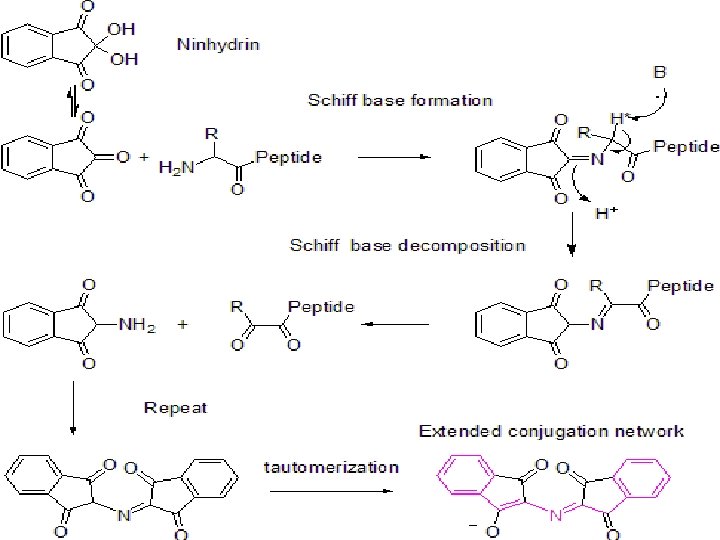
Reaction Mechanism ninhydrin (or triketohydrindene hydrate) is a strong oxidizing agent amines (including all physiological α-amino acids) react with it cause oxidative deamination of α-amino acid function products: aldehyde, ammonia, carbon dioxide, hydrindantin
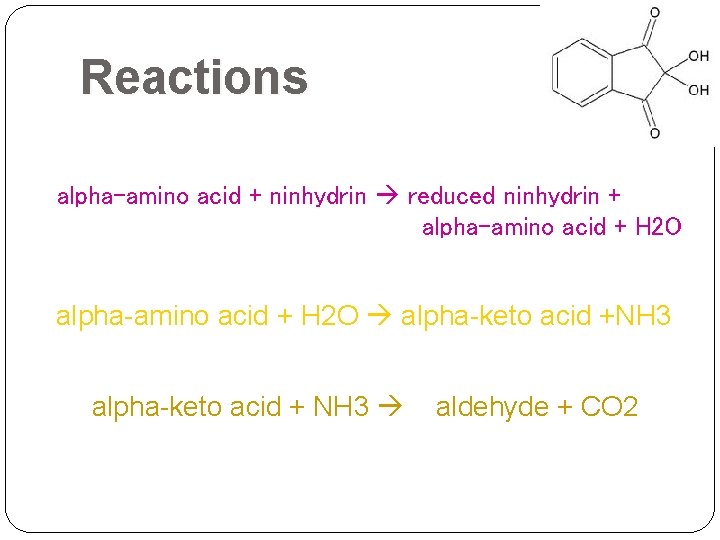
Reactions alpha-amino acid + ninhydrin reduced ninhydrin + alpha-amino acid + H 2 O alpha-keto acid +NH 3 alpha-keto acid + NH 3 aldehyde + CO 2
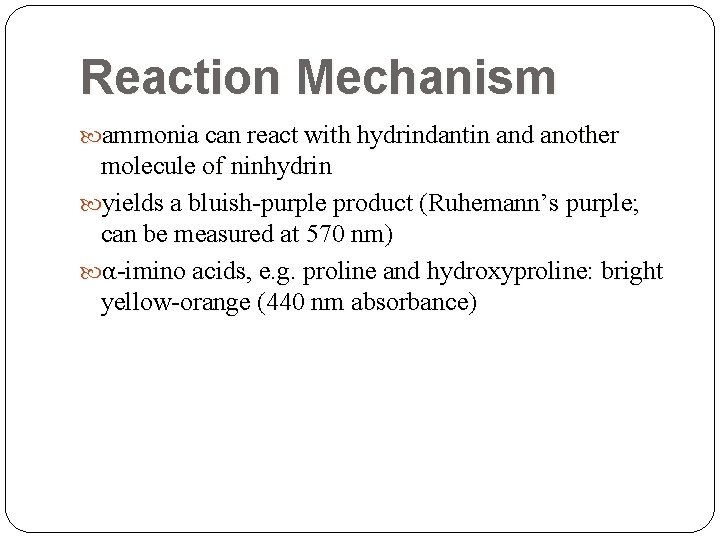
Reaction Mechanism ammonia can react with hydrindantin and another molecule of ninhydrin yields a bluish-purple product (Ruhemann’s purple; can be measured at 570 nm) α-imino acids, e. g. proline and hydroxyproline: bright yellow-orange (440 nm absorbance)
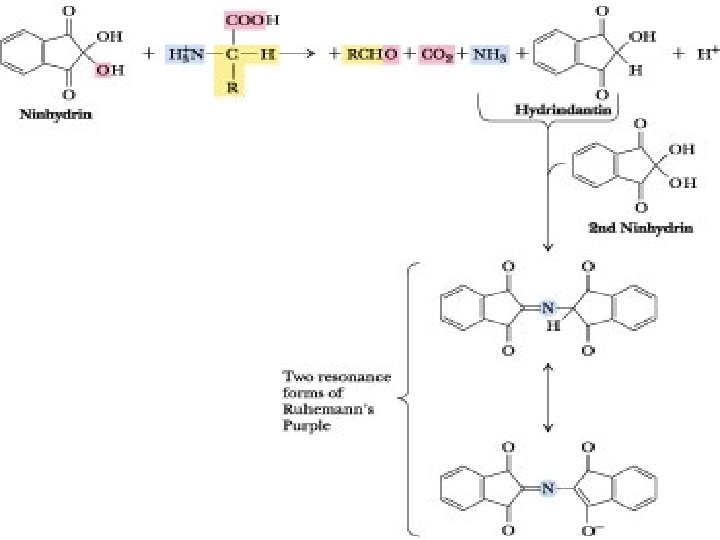
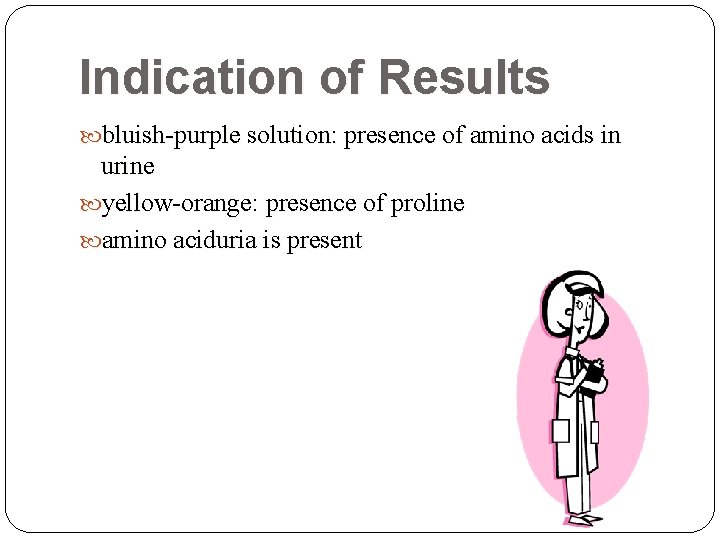
Indication of Results bluish-purple solution: presence of amino acids in urine yellow-orange: presence of proline amino aciduria is present

Amino Aciduria increased levels of amino acid excretion in the urine indicates possible inborn errors of metabolism caused by a specific enzyme deficiency (e. g. Hartnup’s disease, Tyrosinemia)

Millon’s Test Screening Tests for Certain Inborn Errors of Metabolism
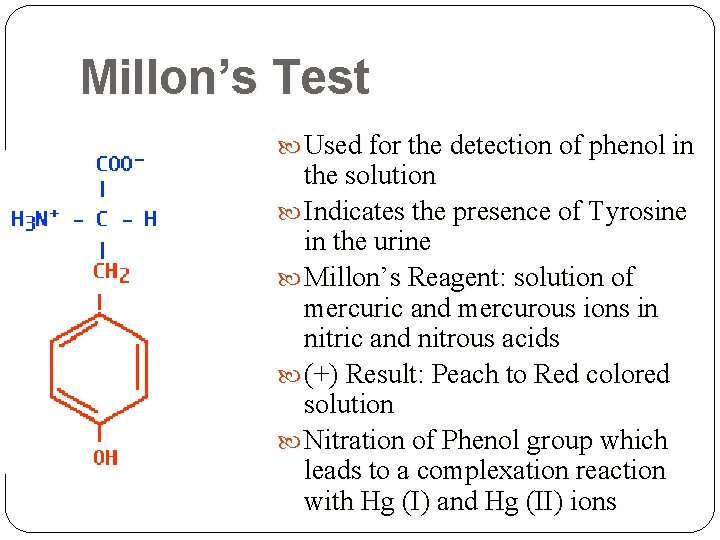
Millon’s Test Used for the detection of phenol in the solution Indicates the presence of Tyrosine in the urine Millon’s Reagent: solution of mercuric and mercurous ions in nitric and nitrous acids (+) Result: Peach to Red colored solution Nitration of Phenol group which leads to a complexation reaction with Hg (I) and Hg (II) ions

Methodology Add 1 drop of Millon’s reagent to 1 m. L urine Water bath for 15 minutes
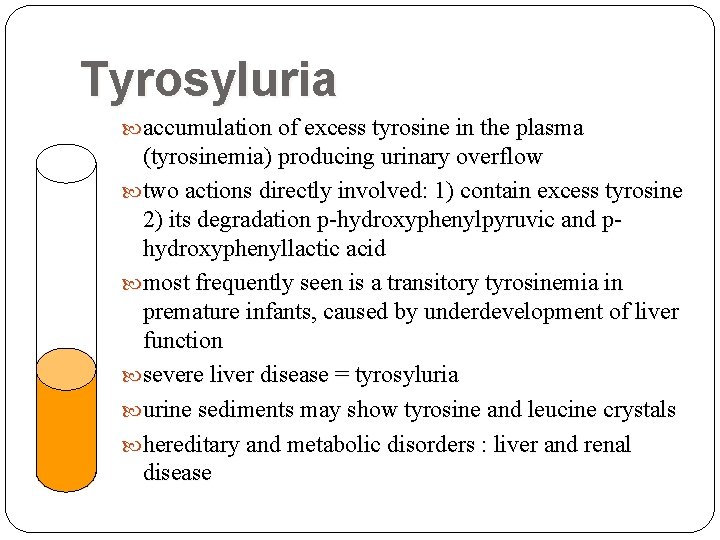
Tyrosyluria accumulation of excess tyrosine in the plasma (tyrosinemia) producing urinary overflow two actions directly involved: 1) contain excess tyrosine 2) its degradation p-hydroxyphenylpyruvic and phydroxyphenyllactic acid most frequently seen is a transitory tyrosinemia in premature infants, caused by underdevelopment of liver function severe liver disease = tyrosyluria urine sediments may show tyrosine and leucine crystals hereditary and metabolic disorders : liver and renal disease
Paper Chromatography of Amino Acids
Paper Chromatography method for testing the purity of compounds and identifying substances useful technique because it is relatively quick and requires small quantities of material used for screening patients for possible amino acid abnormalities
Paper Chromatography substances are distributed between a stationary phase and a mobile phase standard mixtures of pure amino acids are run at the same time as the unknown (urine sample) positions of the spots are compared preliminary preparation of urine samples: to remove salts and proteins
Methodology Spot 6 – 7 x, Dry in between applications Spot as small as possible (too big spots – samples overlap & influence movement of solute) Place in chromatographic jar Solvent front top of paper Air dry Spray with Ninhydrin Place in hot plate until spots are visible Measure distance travelled by amino acids


Solvent system: ETHANOL: NH 4 OH: H 2 O (8: 1: 1)
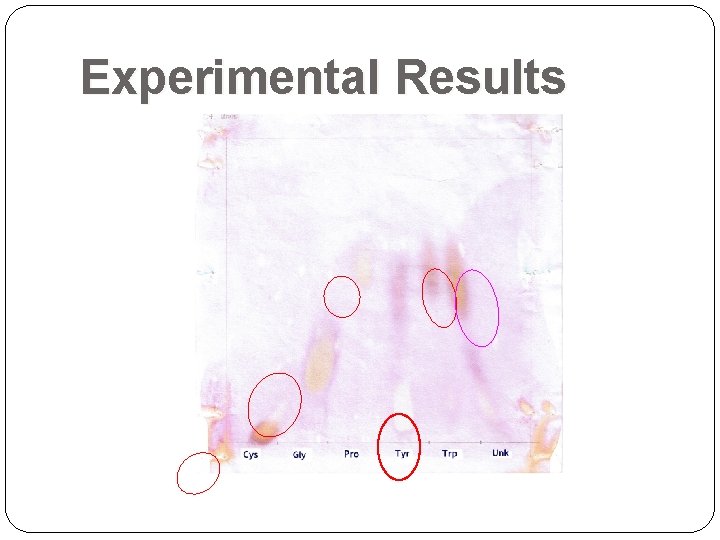
Experimental Results
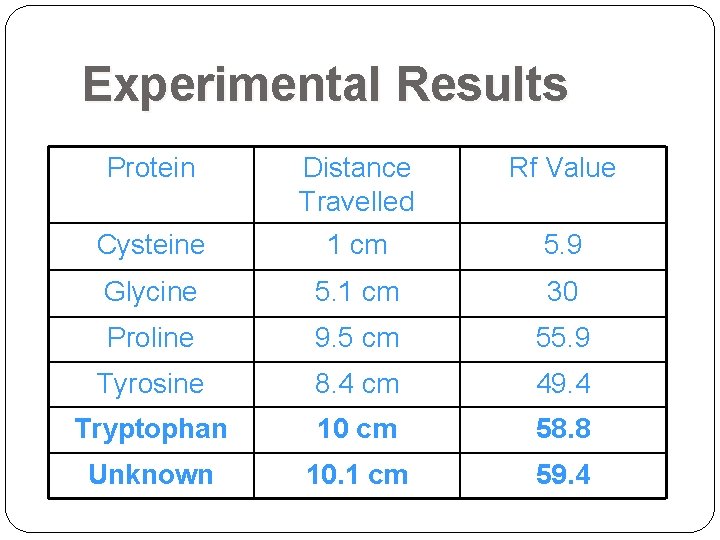
Experimental Results Protein Distance Travelled Rf Value Cysteine 1 cm 5. 9 Glycine 5. 1 cm 30 Proline 9. 5 cm 55. 9 Tyrosine 8. 4 cm 49. 4 Tryptophan 10 cm 58. 8 Unknown 10. 1 cm 59. 4

Experimental Results Distance traveled by solvent = 17 cm ran off the paper: margins were disregarded Unknown = Tryptophan
- Slides: 104