Experiment 1 Obtain a tube of water and

Experiment 1. Obtain a tube of water and a straw. 2. Exhale deeply through the straw into the water. 3. Make observations. 4. Why might your observations be different than the people near you?
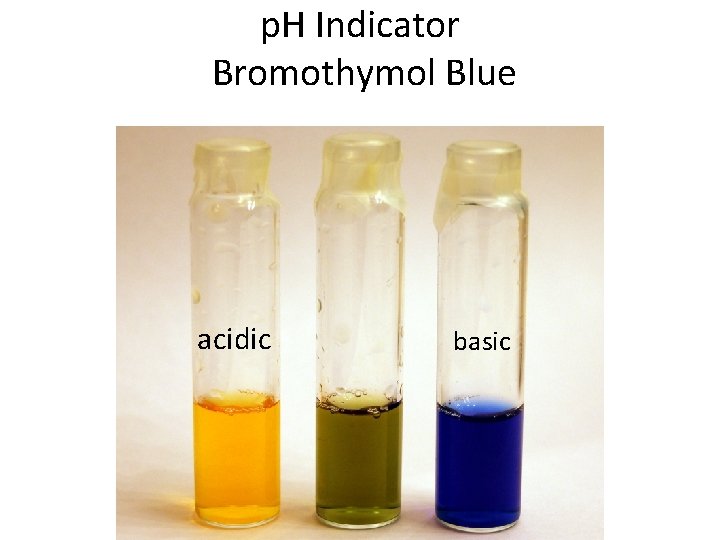
p. H Indicator Bromothymol Blue acidic basic
![p. H scale p. H = -log [H+] a [H+]=1 • 10 -14 http: p. H scale p. H = -log [H+] a [H+]=1 • 10 -14 http:](http://slidetodoc.com/presentation_image_h2/b9938640b7b3e35a1aa19e91a8a74f16/image-3.jpg)
p. H scale p. H = -log [H+] a [H+]=1 • 10 -14 http: //cwx. prenhall. com/bookbind/pubbooks/hillchem 3/medialib/media_portfolio/15. html
![Example p. H = -log [H+] What would be the p. H of 0. Example p. H = -log [H+] What would be the p. H of 0.](http://slidetodoc.com/presentation_image_h2/b9938640b7b3e35a1aa19e91a8a74f16/image-4.jpg)
Example p. H = -log [H+] What would be the p. H of 0. 01 M solution of HCl (like your stomach acid)? p. H = -log [H+] p. H = - -2 p. H = 2
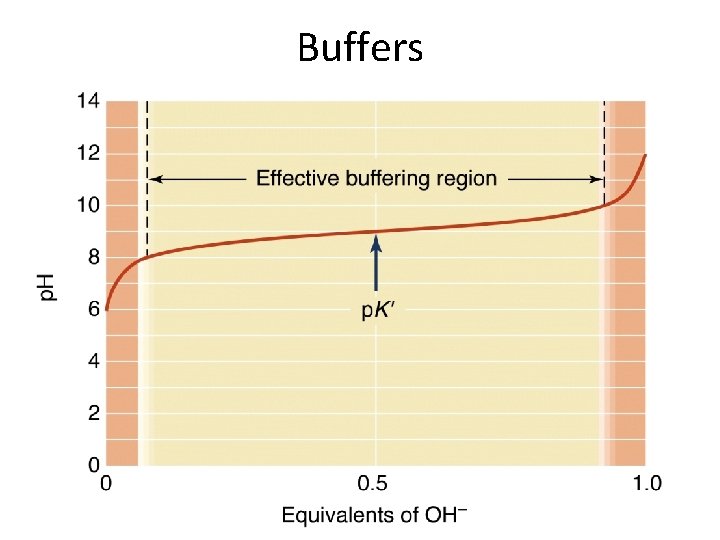
Buffers
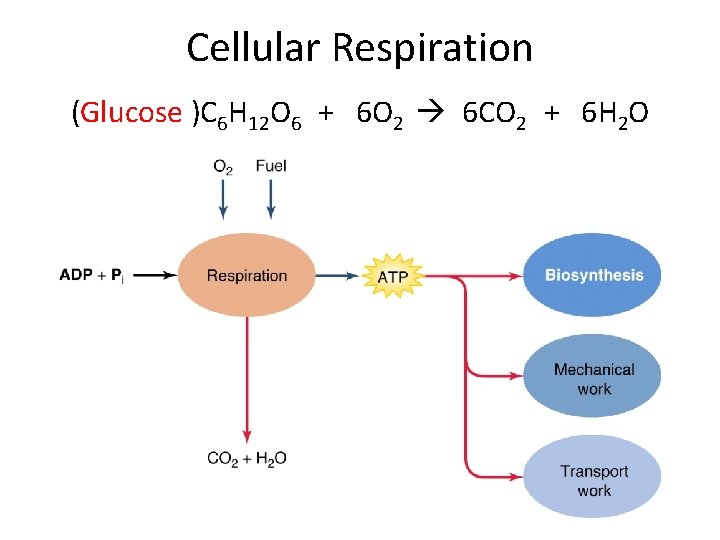
Cellular Respiration (Glucose )C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

Inside the cell
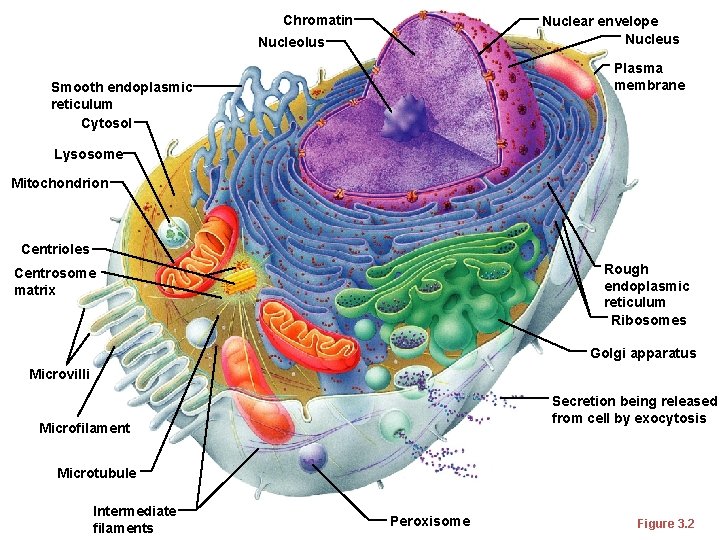
Chromatin Nuclear envelope Nucleus Nucleolus Plasma membrane Smooth endoplasmic reticulum Cytosol Lysosome Mitochondrion Centrioles Rough endoplasmic reticulum Ribosomes Centrosome matrix Golgi apparatus Microvilli Secretion being released from cell by exocytosis Microfilament Microtubule Intermediate filaments Peroxisome Figure 3. 2
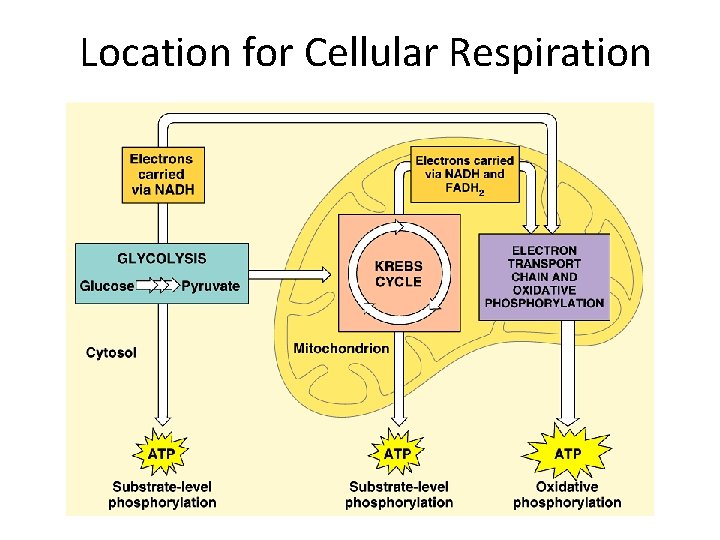
Location for Cellular Respiration
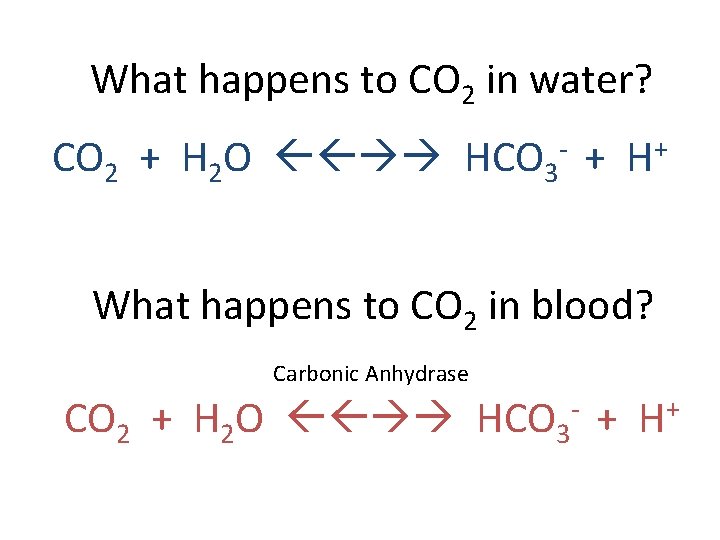
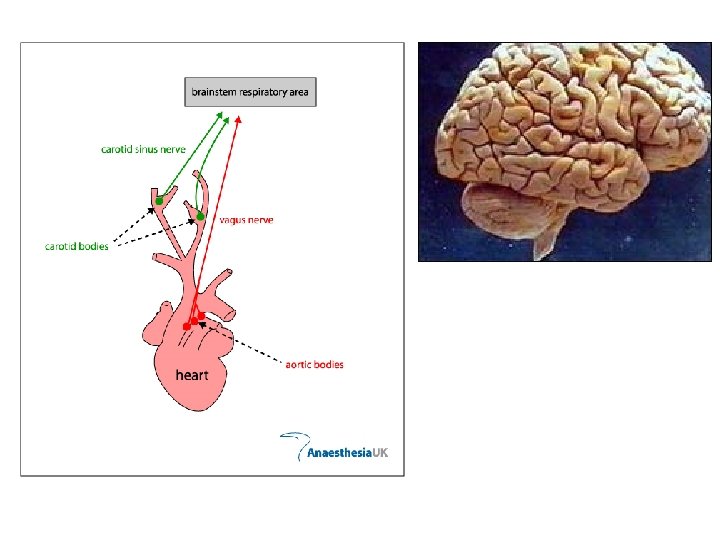
What happens to CO 2 in water? CO 2 + H 2 O HCO 3 - + H+ What happens to CO 2 in blood? Carbonic Anhydrase CO 2 + H 2 O HCO 3 - + H+
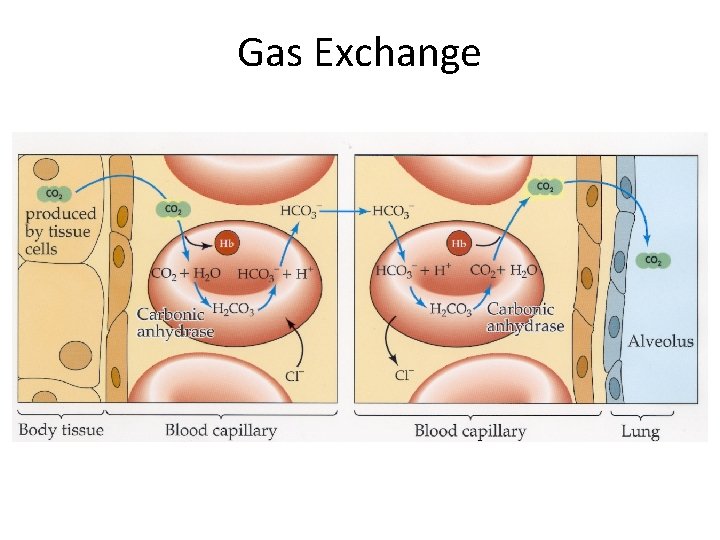
Gas Exchange


CO 2
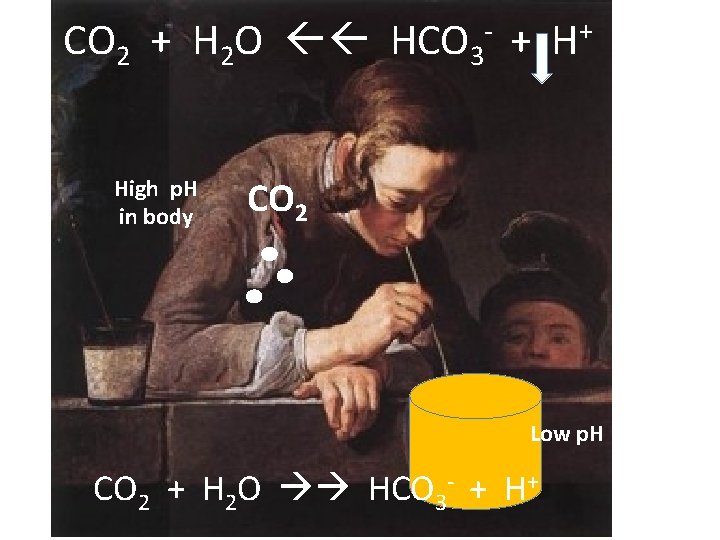
CO 2 + H 2 O HCO 3 - + H+ High p. H in body CO 2 Low p. H CO 2 + H 2 O HCO 3 - + H+
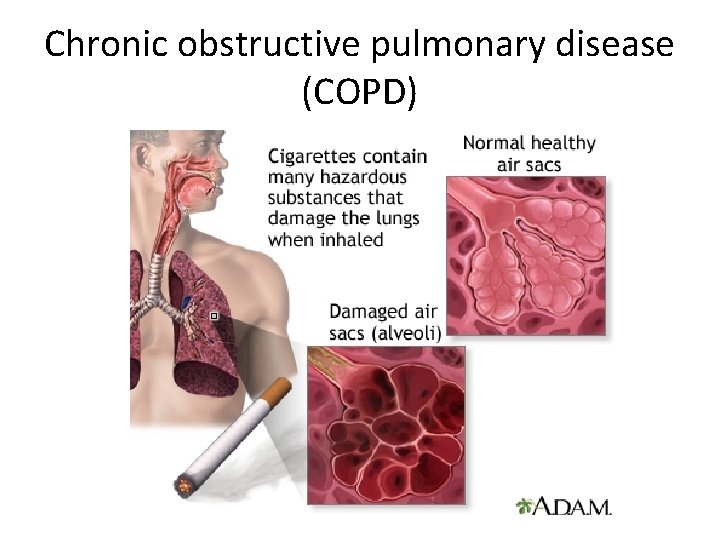
Chronic obstructive pulmonary disease (COPD)
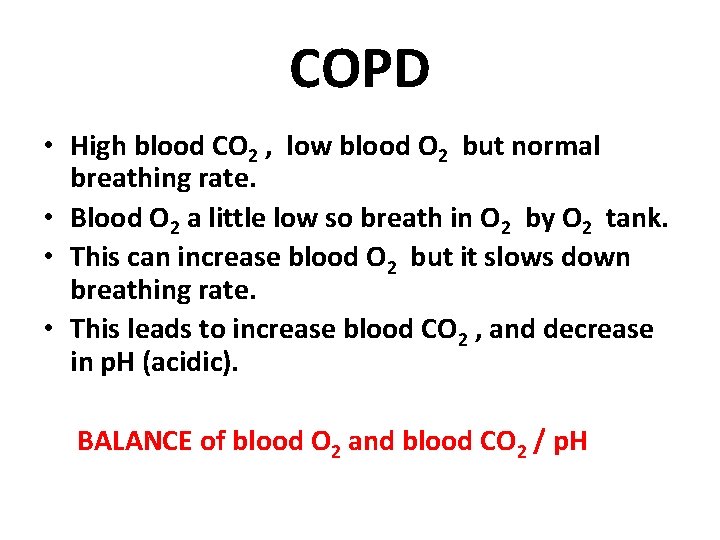
COPD • High blood CO 2 , low blood O 2 but normal breathing rate. • Blood O 2 a little low so breath in O 2 by O 2 tank. • This can increase blood O 2 but it slows down breathing rate. • This leads to increase blood CO 2 , and decrease in p. H (acidic). BALANCE of blood O 2 and blood CO 2 / p. H
- Slides: 17