Experience on Early Feasibility Studies US Industry View

Experience on Early Feasibility Studies: US Industry View Robert Thatcher President & CEO, 4 C Medical Technologies, Inc.

Robert Thatcher Employee and/or Stockholder: 4 C Medical Technologies, Inc.
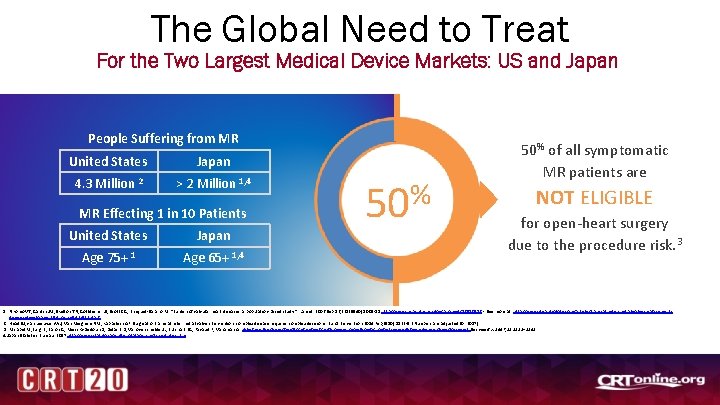
The Global Need to Treat For the Two Largest Medical Device Markets: US and Japan People Suffering from MR United States Japan 4. 3 Million 2 > 2 Million 1, 4 MR Effecting 1 in 10 Patients United States Japan Age 75+ 1 Age 65+ 1, 4 % 50 50% of all symptomatic MR patients are NOT ELIGIBLE for open-heart surgery due to the procedure risk. 3 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. “Burden of valvular heart diseases: a population-based study. ” Lancet. 2006 Sep 16; 368(9540): 1005 -11. http: //www. ncbi. nlm. nih. gov/pubmed/16980116 - See more at: http: //www. dicardiology. com/article/transcatheter-mitral-valve-replacementdevices-development#sthash. e. Sd. Udl 2 E. dpuf 2. Head SJ, van Leeuwen WJ, Van Mieghem NM, Kappetein AP. Surgical or transcatheter mitral valve intervention: complex disease requires complex decisions. Euro. Intervention. 2014 Feb; 9(10): 1133 -5 (Numbers are adjusted for 2017) 3. Mirabel M, lung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007; 28: 1358– 1365 4. Japan Statistics Bureau. 2017 http: //www. stat. go. jp/english/data/jinsui/tsuki/index. htm
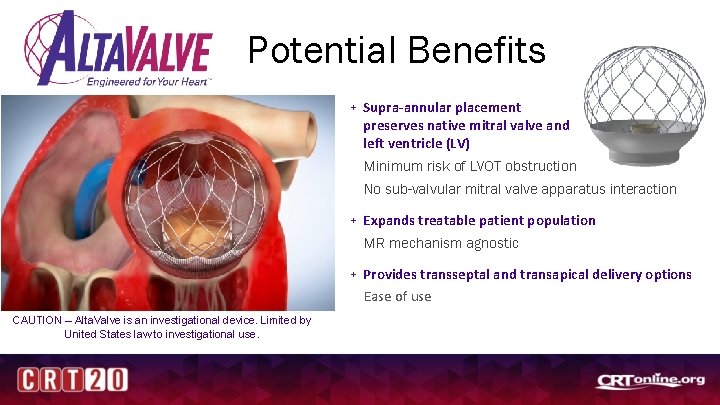
Potential Benefits + Supra-annular placement preserves native mitral valve and left ventricle (LV) Minimum risk of LVOT obstruction No sub-valvular mitral valve apparatus interaction + Expands treatable patient population MR mechanism agnostic + CAUTION – Alta. Valve is an investigational device. Limited by United States law to investigational use. Provides transseptal and transapical delivery options Ease of use
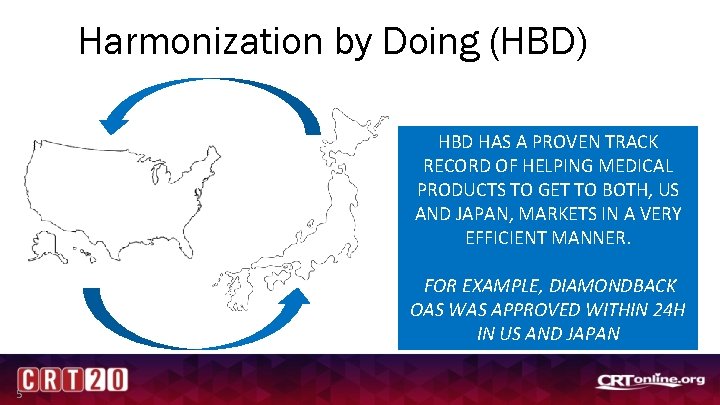
Harmonization by Doing (HBD) HBD HAS A PROVEN TRACK RECORD OF HELPING MEDICAL PRODUCTS TO GET TO BOTH, US AND JAPAN, MARKETS IN A VERY EFFICIENT MANNER. FOR EXAMPLE, DIAMONDBACK OAS WAS APPROVED WITHIN 24 H IN US AND JAPAN 5
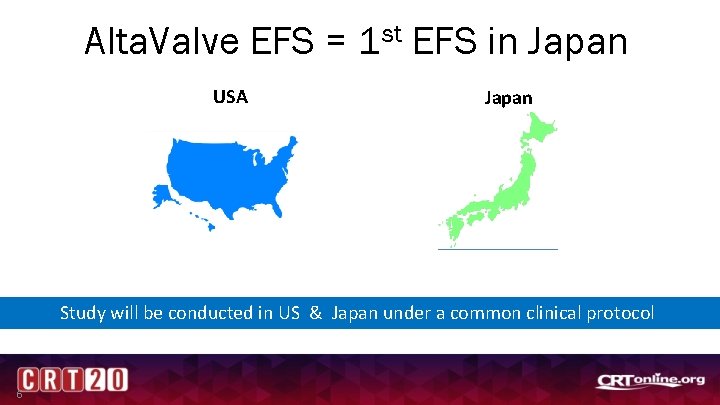
Alta. Valve EFS = 1 st EFS in Japan USA Japan Study will be conducted in US & Japan under a common clinical protocol 6
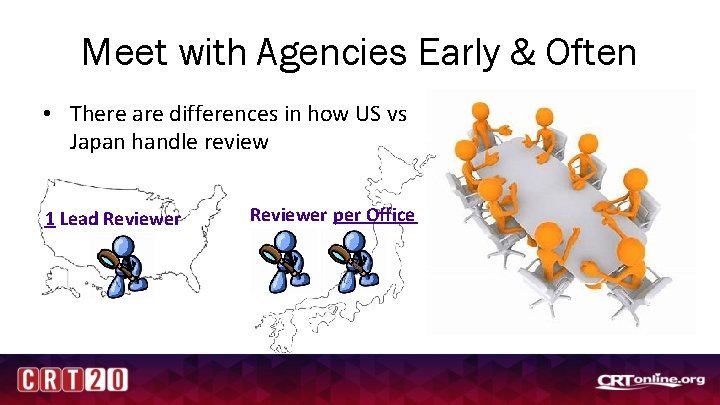
Meet with Agencies Early & Often • There are differences in how US vs Japan handle review 1 Lead Reviewer per Office
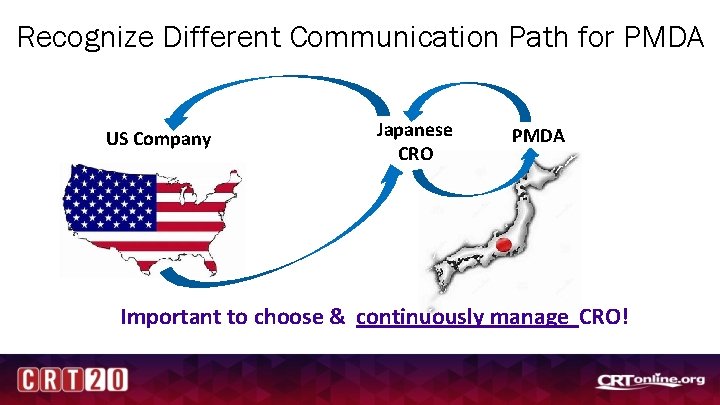
Recognize Different Communication Path for PMDA US Company Japanese CRO PMDA Important to choose & continuously manage CRO!

Clinical Operations Challenges in Japan – Example #1 Japan historically evaluates technologies that are commercially available in other countries EFS devices are 1 st generation devices and require intense training where physicians need to be comfortable with many unknowns Challenge: Sponsors take on responsibility to train the sites and work through lessons learned along the way. CE Mark 2008 FDA approval 2013 PMDA approval 2018

Clinical Operations Challenges in Japan – Example #2 Notification No. 0329, No. 14, March 29, 2013 Reporting to the PMDA about Defects Pertaining to Machinery and Equipment Identified during Clinical Trials “Events that cannot be expected from the Investigator’s Brochure for a medical device” means events that were not described in the Investigator’s Brochure for a medical device or events not conforming to the data on characteristics, severity, or incidence in the Brochure if the event was described. For example, a severe event that was unexpected because a less severe event was described in the Investigator’s Brochure for a medical device is subject to reporting. In Japan, determination of adverse events being expected is based on events listed in the Investigators Brochure, verbatim Opportunity: PMDA may consider updating Ordinance for all futures studies to allow the informed consent and other study materials to be used for determination of AE expectedness, and the verbatim requirement could then be removed

Alta. Valve Transapical First In Human Experience Pre-Procedure Echo Post-Procedure LV Gram
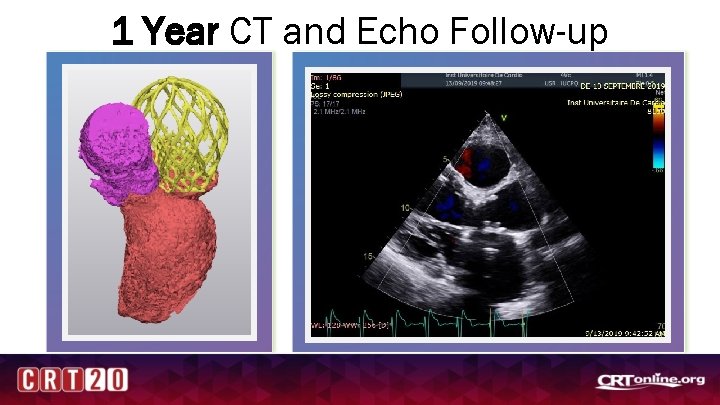
1 Year CT and Echo Follow-up
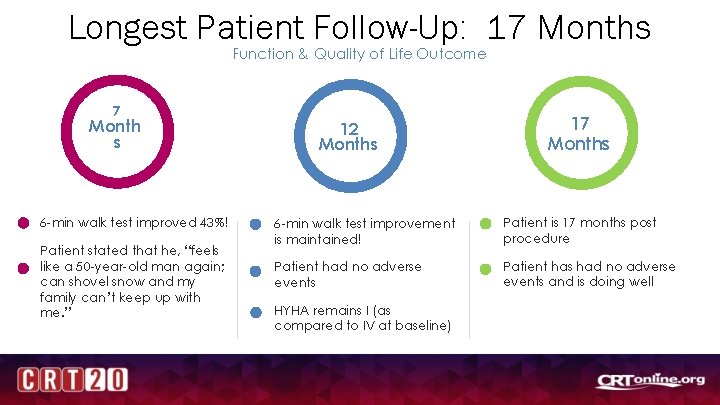
Longest Patient Follow-Up: 17 Months Function & Quality of Life Outcome 7 Month s 6 -min walk test improved 43%! Patient stated that he, “feels like a 50 -year-old man again; can shovel snow and my family can’t keep up with me. ” 12 Months 17 Months 6 -min walk test improvement is maintained! Patient is 17 months post procedure Patient had no adverse events Patient has had no adverse events and is doing well HYHA remains I (as compared to IV at baseline)
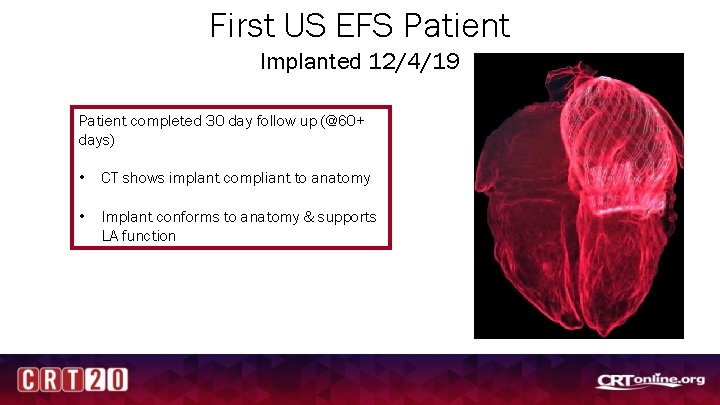
First US EFS Patient Implanted 12/4/19 Patient completed 30 day follow up (@60+ days) • CT shows implant compliant to anatomy • Implant conforms to anatomy & supports LA function

Conclusion With the help of HBD, globalization of clinical trials will lead to: • Elimination of repetitive clinical studies - Hence, reduction in time and cost • Global learning of patient treatment outcomes • Faster approval in both US and Japan • Earlier access to technology for Japanese patients who historically wait longer as compared to ROW - New technology - Next generation versions of technology

Thank you!
- Slides: 16