Expanding HIV testing and the use of ARVs









- Slides: 29

Expanding HIV testing and the use of ARVs for treatment and prevention Getting to 15 million by 2015… and thinking beyond Gottfried Hirnschall MD, MPH Department of HIV/AIDS, World Health Organization July 26, 2012

Questions for today • Can we reach 15 million by 2015? • Is 15 million enough to achieve optimal impact on treatment and prevention? • What strategic choices can be made? What are the opportunities to enhance ART program effectiveness and reach?

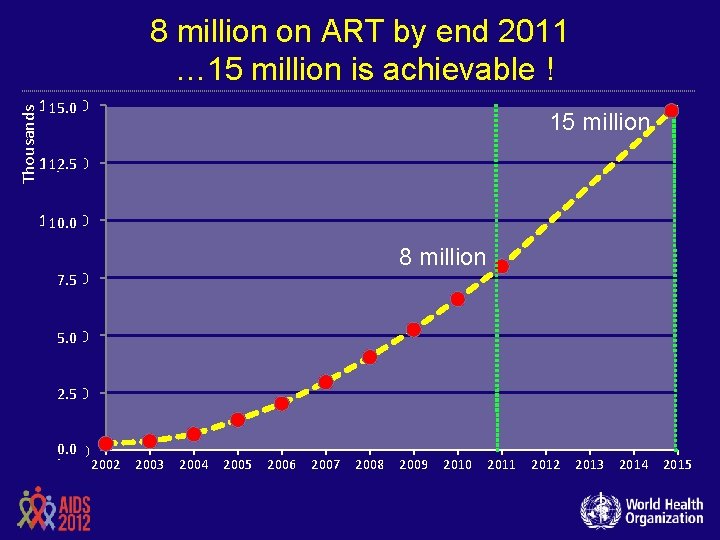
Thousands 8 million on ART by end 2011 … 15 million is achievable ! 15, 000 15 million 12, 500 12. 5 10, 000 10. 0 7, 500 7. 5 8 million 5, 000 5. 0 2, 500 2. 5 0. 0 , 000 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015

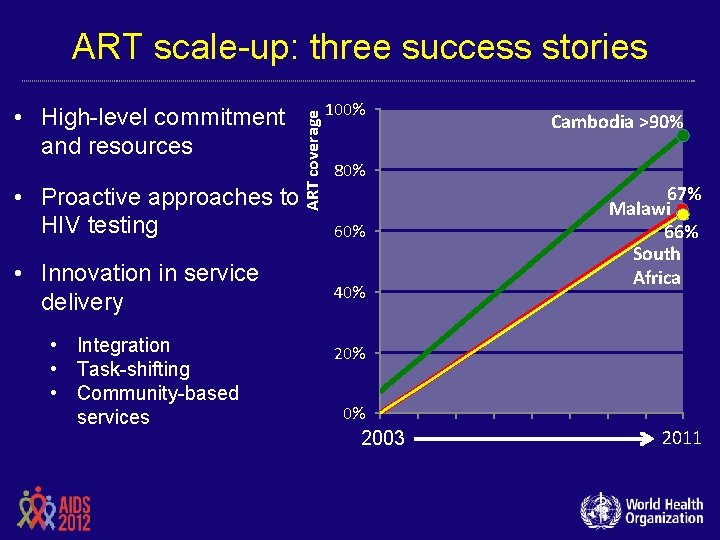
• High-level commitment and resources • Proactive approaches to HIV testing • Innovation in service delivery • Integration • Task-shifting • Community-based services ART coverage ART scale-up: three success stories 100% Cambodia >90% 80% 60% 40% 67% Malawi 66% South Africa 20% 0% 2003 2011

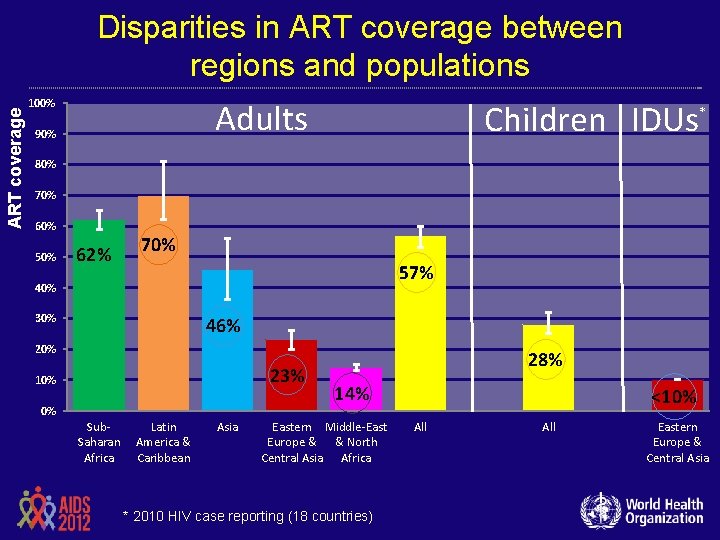
ART coverage Disparities in ART coverage between regions and populations 100% Adults 90% Children IDUs* 80% 70% 60% 50% 62% 70% 57% 40% 30% 46% 20% 23% 10% 0% Sub. Saharan Africa Latin America & Caribbean Asia 28% 14% Eastern Middle-East Europe & & North Central Asia Africa * 2010 HIV case reporting (18 countries) <10% All Eastern Europe & Central Asia

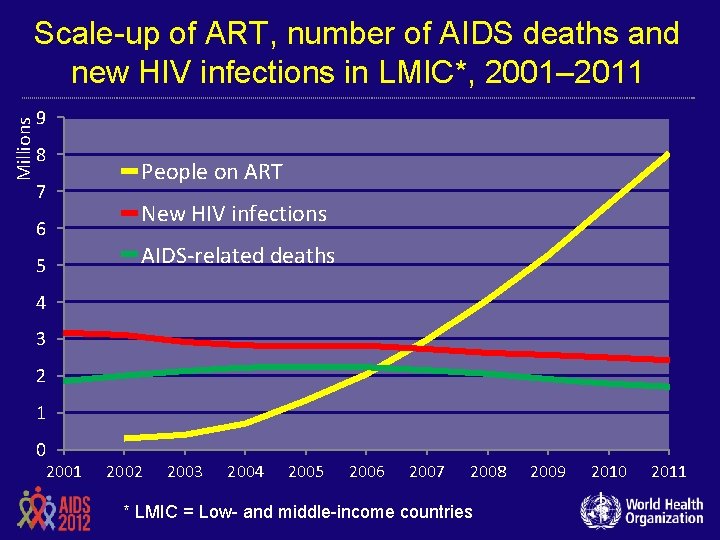
Millions Scale-up of ART, number of AIDS deaths and new HIV infections in LMIC*, 2001– 2011 9 8 7 6 5 People on ART New HIV infections AIDS-related deaths 4 3 2 1 0 2001 2002 2003 2004 2005 2006 2007 2008 * LMIC = Low- and middle-income countries 2009 2010 2011

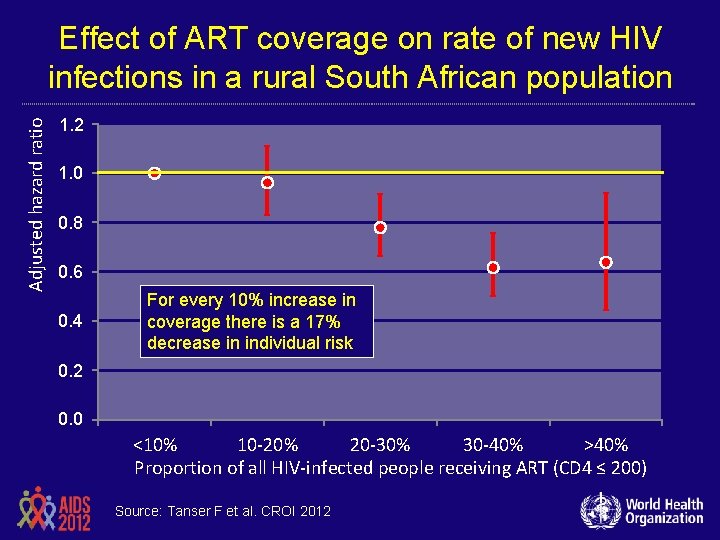
Adjusted hazard ratio Effect of ART coverage on rate of new HIV infections in a rural South African population 01 1. 2 1. 0 01 0. 8 01 0. 6 01 0. 4 00 For every 10% increase in coverage there is a 17% decrease in individual risk 0. 2 00 0. 0 00 <10% 10 -20% 20 -30% 30 -40% >40% Proportion of all HIV-infected people receiving ART (CD 4 ≤ 200) Source: Tanser F et al. CROI 2012

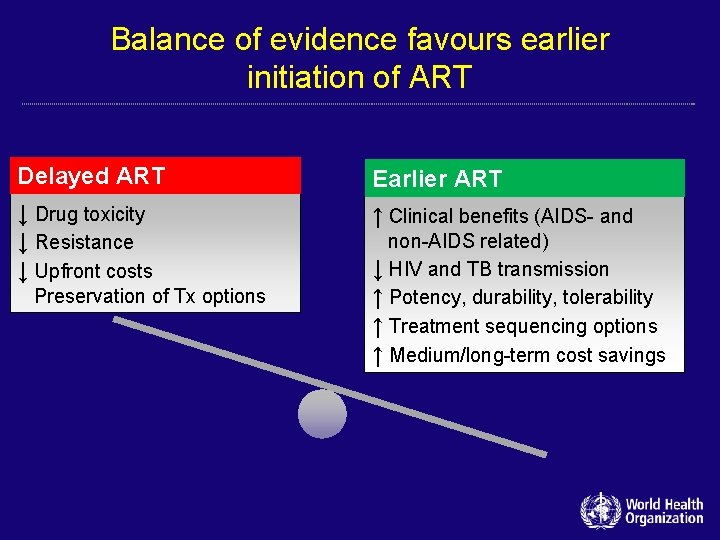
Balance of evidence favours earlier initiation of ART Delayed ART Earlier ART ↓ Drug toxicity ↓ Resistance ↓ Upfront costs ↑ Clinical benefits (AIDS- and Preservation of Tx options non-AIDS related) ↓ HIV and TB transmission ↑ Potency, durability, tolerability ↑ Treatment sequencing options ↑ Medium/long-term cost savings

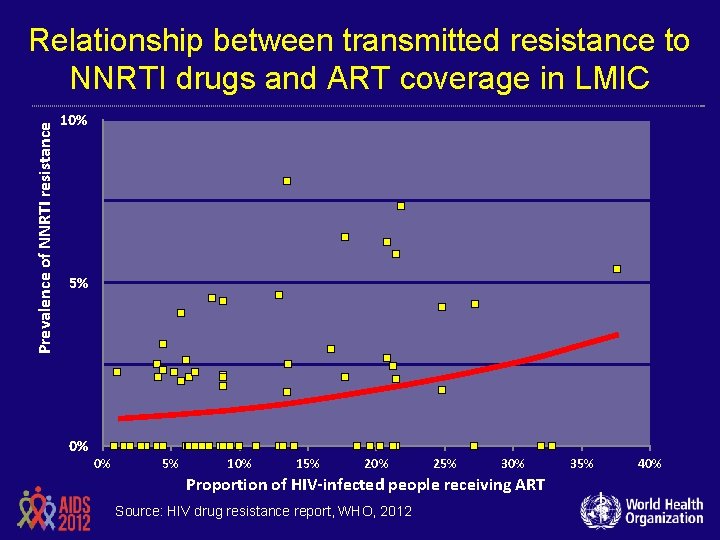
Prevalence of NNRTI resistance Relationship between transmitted resistance to NNRTI drugs and ART coverage in LMIC 10% 5% 0% 0% 5% 10% 15% 20% 25% 30% Proportion of HIV-infected people receiving ART Source: HIV drug resistance report, WHO, 2012 35% 40%

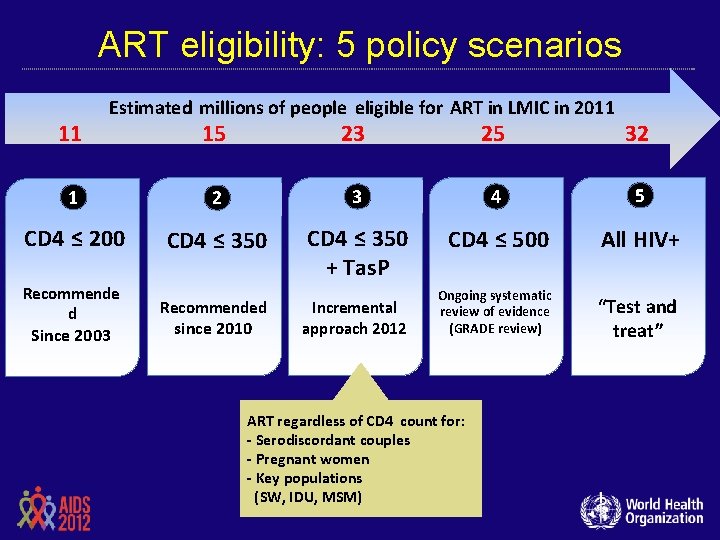
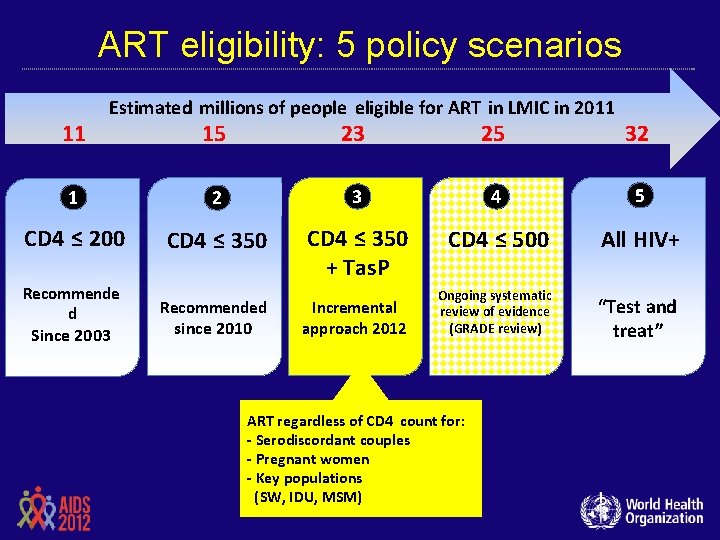
ART eligibility: 5 policy scenarios 11 Estimated millions of people eligible for ART in LMIC in 2011 15 23 25 32 1 2 3 4 5 CD 4 ≤ 200 CD 4 ≤ 350 + Tas. P CD 4 ≤ 500 All HIV+ Ongoing systematic review of evidence (GRADE review) “Test and treat” Recommende d Since 2003 Recommended since 2010 Incremental approach 2012 ART regardless of CD 4 count for: - Serodiscordant couples - Pregnant women - Key populations (SW, IDU, MSM)

ART eligibility: 5 policy scenarios 11 Estimated millions of people eligible for ART in LMIC in 2011 15 23 25 32 1 2 3 4 5 CD 4 ≤ 200 CD 4 ≤ 350 + Tas. P CD 4 ≤ 500 All HIV+ Ongoing systematic review of evidence (GRADE review) “Test and treat” Recommende d Since 2003 Recommended since 2010 Incremental approach 2012 ART regardless of CD 4 count for: - Serodiscordant couples - Pregnant women - Key populations (SW, IDU, MSM)

WHO’s ARV-related guidance in 2012 Treatment as Prevention (Tas. P) • Recommendation for Tas. P in sero-discordant couples • Consider lifelong ART for pregnant women (“B/B+”) • Explore use of Tas. P in key populations (SW, IDU, MSM) Pre-Exposure Prophylaxis (Pr. EP) • Recommendation for demonstration projects in sero-discordant couples and MSM

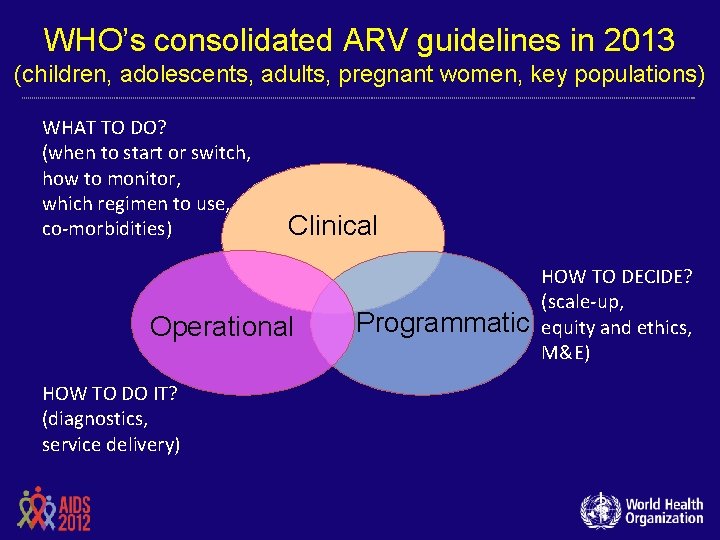
WHO’s consolidated ARV guidelines in 2013 (children, adolescents, adults, pregnant women, key populations) WHAT TO DO? (when to start or switch, how to monitor, which regimen to use, co-morbidities) Clinical Operational HOW TO DO IT? (diagnostics, service delivery) Programmatic HOW TO DECIDE? (scale-up, equity and ethics, M&E)

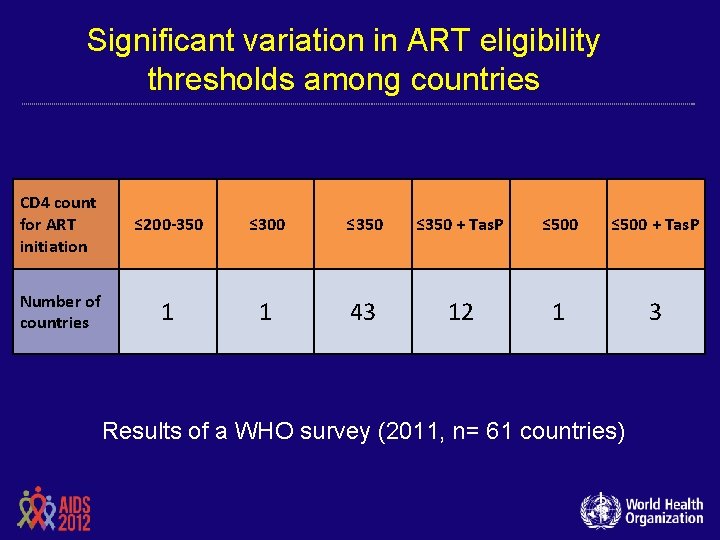
Significant variation in ART eligibility thresholds among countries CD 4 count for ART initiation ≤ 200 -350 ≤ 300 ≤ 350 + Tas. P ≤ 500 + Tas. P Number of countries 1 1 43 12 1 3 Results of a WHO survey (2011, n= 61 countries)

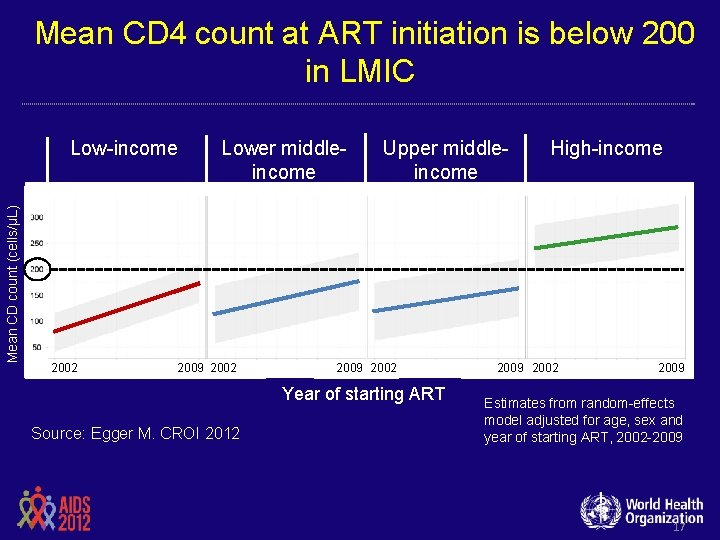
Mean CD 4 count at ART initiation is below 200 in LMIC Mean CD count (cells/µL) Low-income 2002 Lower middleincome 2009 2002 Upper middleincome 2009 2002 Year of starting ART Source: Egger M. CROI 2012 High-income 2009 2002 2009 Estimates from random-effects model adjusted for age, sex and year of starting ART, 2002 -2009 17

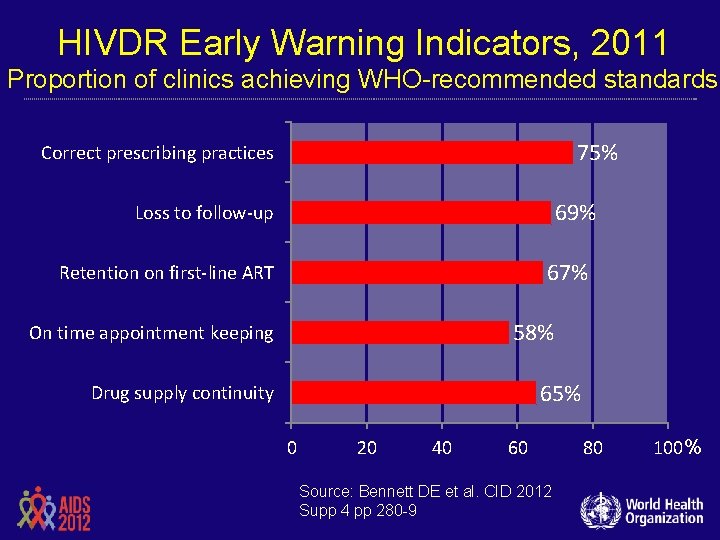
HIVDR Early Warning Indicators, 2011 Proportion of clinics achieving WHO-recommended standards 75% Correct prescribing practices 69% Loss to follow-up 67% Retention on first-line ART 58% On time appointment keeping 65% Drug supply continuity 0 20 40 60 Source: Bennett DE et al. CID 2012 Supp 4 pp 280 -9 80 100 %

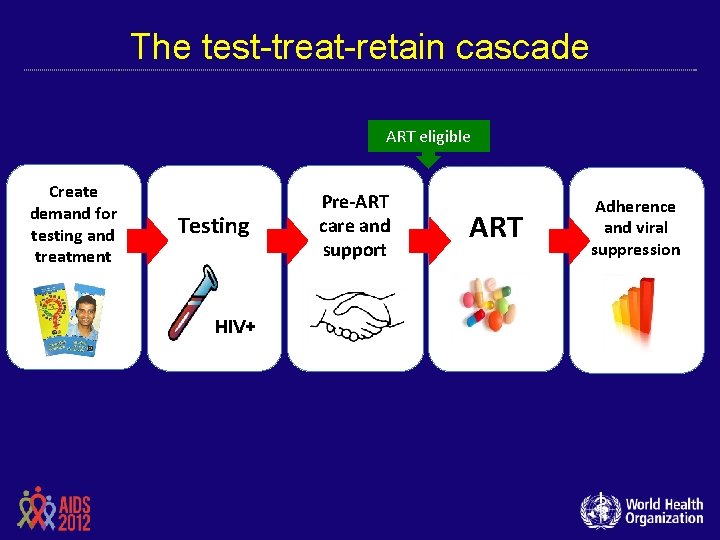
The test-treat-retain cascade ART eligible Create demand for testing and treatment Testing + HIV+ Pre-ART care and support ART Adherence and viral suppression

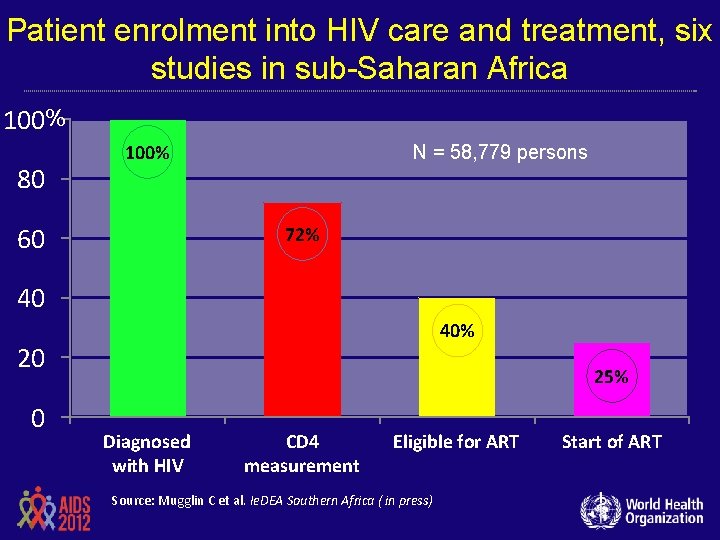
Patient enrolment into HIV care and treatment, six studies in sub-Saharan Africa 100 % 80 100% 60 N = 58, 779 persons 72% 40 40% 20 0 25% Diagnosed with HIV CD 4 measurement Eligible for ART Source: Mugglin C et al. Ie. DEA Southern Africa ( in press) Start of ART

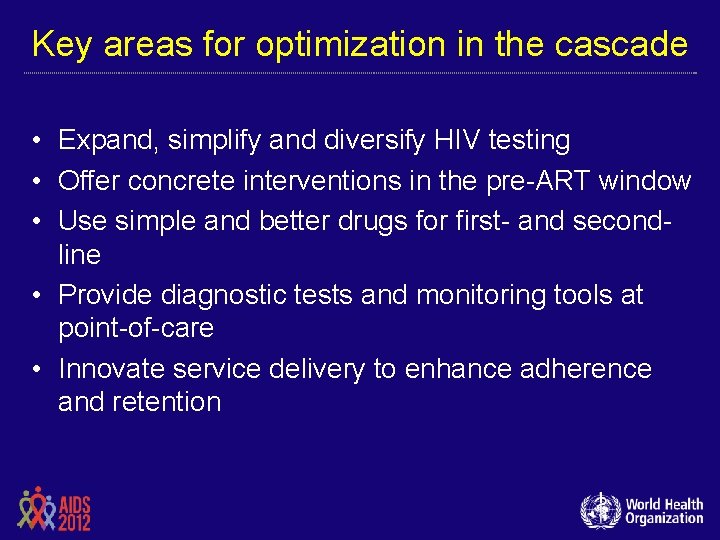
Key areas for optimization in the cascade • Expand, simplify and diversify HIV testing • Offer concrete interventions in the pre-ART window • Use simple and better drugs for first- and secondline • Provide diagnostic tests and monitoring tools at point-of-care • Innovate service delivery to enhance adherence and retention

Provider-initiated testing and counselling (PITC) in Africa Adoption of a policy on PITC § 42/53 countries in Africa have PITC policies 1 2003 -2010 § High PITC acceptance by ANC 2 & TB patients 3, 4 § Most clinical settings in generalized epidemics not routinely offering HIV testing 5 1 Baggaley 2003 - 2004 2005 - 2006 2007 - 2008 2009 - 2010 Date not identified Not adopted Data not available (2012) Bulletin WHO, 2 Etirbet (2004) AIDS Care; Byamugisha (2010) J Int AIDS, 3 WHO, Global TB control (2011), 4 Corneli (2008) IJTBLD, 5 Mac. Pherson (2012) Trop Med.

Scaling up HIV testing in the community §Home-based (door-to-door) § Community § Index-case §Campaigns plus § HTC-plus –malaria, safe water § Non-communicable diseases §Mobile outreach § General populations § Key populations §Workplaces, schools

A potential new approach: self-testing Today § Practiced 'informally' by many health workers 1 § Included in Kenyan National Guidelines § Readily available over the internet and in pharmacies in some countries § Approved by FDA in USA this month Future potential § General population? § Marginalized groups? § Pr. EP? 1 Napierala S, (2011). HIV self-testing among health workers

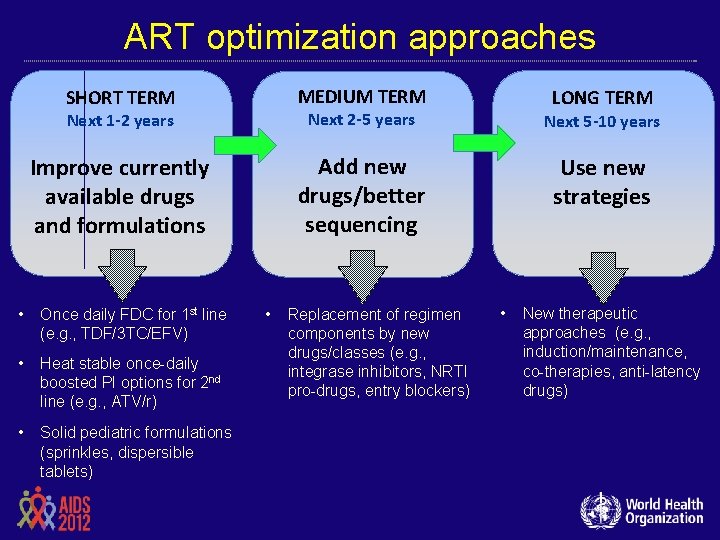
ART optimization approaches SHORT TERM MEDIUM TERM Next 1 -2 years Next 2 -5 years Next 5 -10 years Improve currently available drugs and formulations Add new drugs/better sequencing Use new strategies • Once daily FDC for 1 st line (e. g. , TDF/3 TC/EFV) • Heat stable once-daily boosted PI options for 2 nd line (e. g. , ATV/r) • Solid pediatric formulations (sprinkles, dispersible tablets) • Replacement of regimen components by new drugs/classes (e. g. , integrase inhibitors, NRTI pro-drugs, entry blockers) LONG TERM • New therapeutic approaches (e. g. , induction/maintenance, co-therapies, anti-latency drugs)

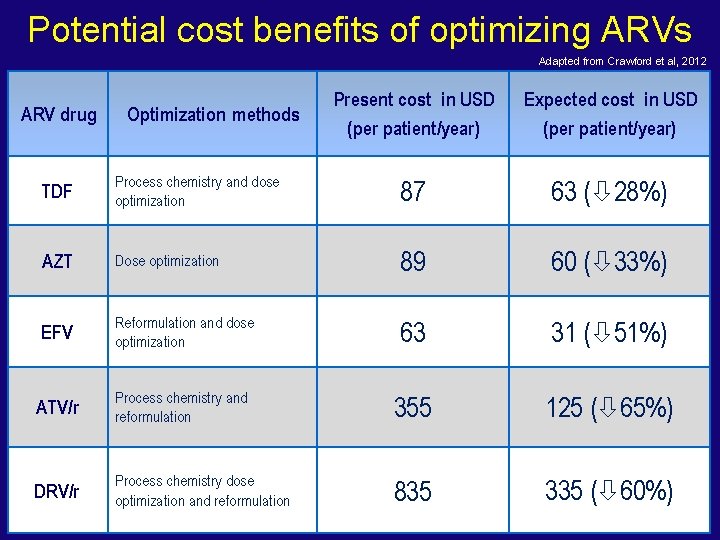
Potential cost benefits of optimizing ARVs Adapted from Crawford et al, 2012 ARV drug Optimization methods Present cost in USD (per patient/year) Expected cost in USD (per patient/year) TDF Process chemistry and dose optimization 87 63 ( 28%) AZT Dose optimization 89 60 ( 33%) EFV Reformulation and dose optimization 63 31 ( 51%) ATV/r Process chemistry and reformulation 355 125 ( 65%) DRV/r Process chemistry dose optimization and reformulation 835 335 ( 60%)

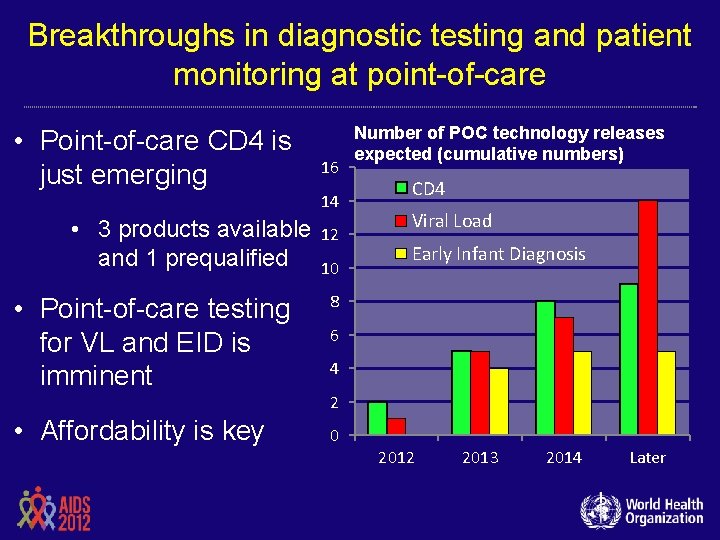
Breakthroughs in diagnostic testing and patient monitoring at point-of-care • Point-of-care CD 4 is just emerging 16 14 • 3 products available and 1 prequalified • Point-of-care testing for VL and EID is imminent • Affordability is key 12 10 Number of POC technology releases expected (cumulative numbers) CD 4 Viral Load Early Infant Diagnosis 8 6 4 2 0 2012 2013 2014 Later

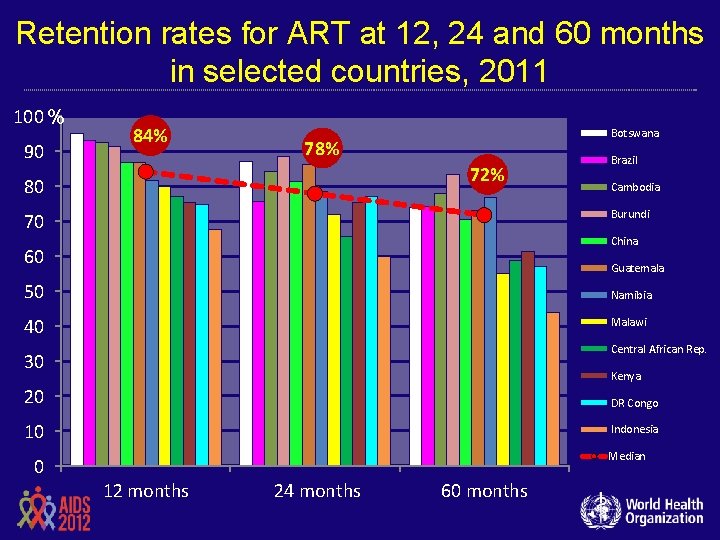
Retention rates for ART at 12, 24 and 60 months in selected countries, 2011 100 % 90 84% Botswana 78% 72% 80 Brazil Cambodia Burundi 70 China 60 Guatemala 50 Namibia 40 Malawi Central African Rep. 30 Kenya 20 DR Congo 10 Indonesia Median 0 12 months 24 months 60 months

Conclusions (1) • Global progress on scale-up of ART has been extraordinary. Countries show the way! 15 million can be reached • Further scale-up must address disparities and inequities (countries, key populations) • With new evidence and new policies, the number of persons eligible for ART will increase • Countries face strategic choices and are already taking advantage of new opportunities (early ART, Tas. P, Pr. EP)

Conclusions (2) • Now is the moment to think and plan beyond the 15 million target • This will require forward-looking policies, more effective and innovative approaches, together with further investments • ARVs for treatment and prevention are a powerful tool towards ending the HIV epidemic

Acknowledgements Rachel Baggaley Andrew Ball Michel Beusenberg Txema Garcia Calleja Wafaa El-Sadr Charles Flexner Nathan Ford Reuben Granich Ian Grubb Tim Hallett Tony Harries Ying-Ru Lo Jos Perriens Yves Souteyrand John Stover Frank Tanser Bernhard Schwartländer Stefano Vella Marco Vitoria Gundo Weiler