Expanded Pr EP implementation across Australia Expanded implementation













- Slides: 22

Expanded Pr. EP implementation across Australia Expanded implementation of Pr. EP across Australia 1

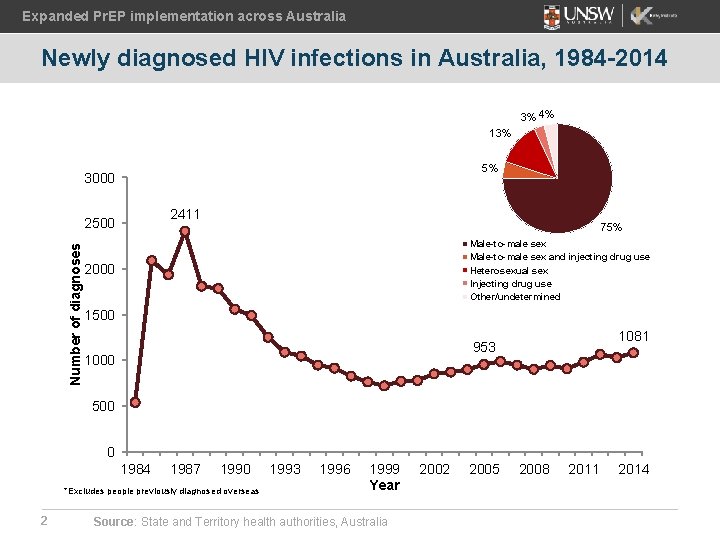
Expanded Pr. EP implementation across Australia Newly diagnosed HIV infections in Australia, 1984 -2014 3% 4% 13% 5% 3000 2411 Number of diagnoses 2500 75% Male-to-male sex and injecting drug use Heterosexual sex Injecting drug use Other/undetermined 2000 1500 1081 953 1000 500 0 1984 1987 1990 *Excludes people previously diagnosed overseas 2 1993 1996 1999 Year Source: State and Territory health authorities, Australia 2002 2005 2008 2011 2014

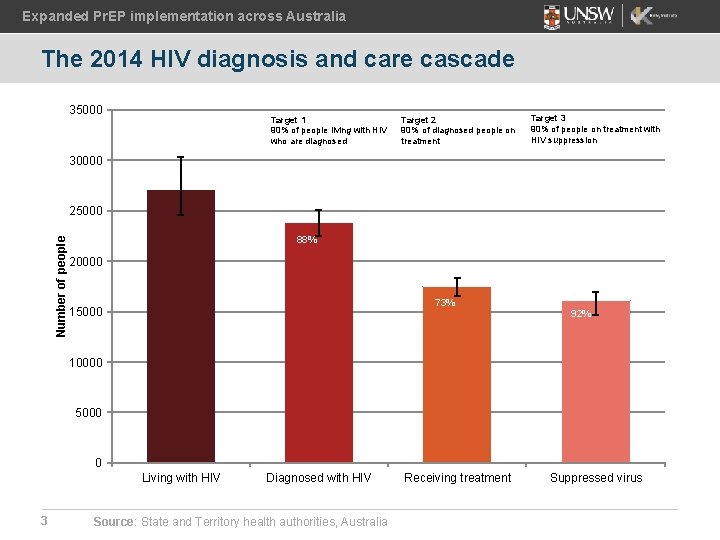
Expanded Pr. EP implementation across Australia The 2014 HIV diagnosis and care cascade 35000 Target 1 90% of people living with HIV who are diagnosed Target 2 90% of diagnosed people on treatment Target 3 90% of people on treatment with HIV suppression 30000 Number of people 25000 88% 20000 73% 15000 92% 10000 5000 0 Living with HIV 3 Diagnosed with HIV Source: State and Territory health authorities, Australia Receiving treatment Suppressed virus

Expanded Pr. EP implementation across Australia Early research on Pr. EP • • 4 Use of Pr. EP among men who have sex with men (MSM) (behavioural surveillance data) • first record of informal use of Pr. EP by MSM (Zablotska et al. JAIDS 2012) • during 2011 -2015, Pr. EP use fluctuated around 2. 5% of gay community-attached men, but increased from 3% to 10% among high-risk men Pr. EP awareness and acceptability (cross-sectional behavioural surveys) • Pr. EPARE study (2012): 27% of HIV negative men were aware about Pr. EP and willing to take it (Holt et al. JAIDS , 2014) • TORCH study (2013): 71% of high risk men were willing to take Pr. EP (high risk defined as in Australian Pr. EP guidelines

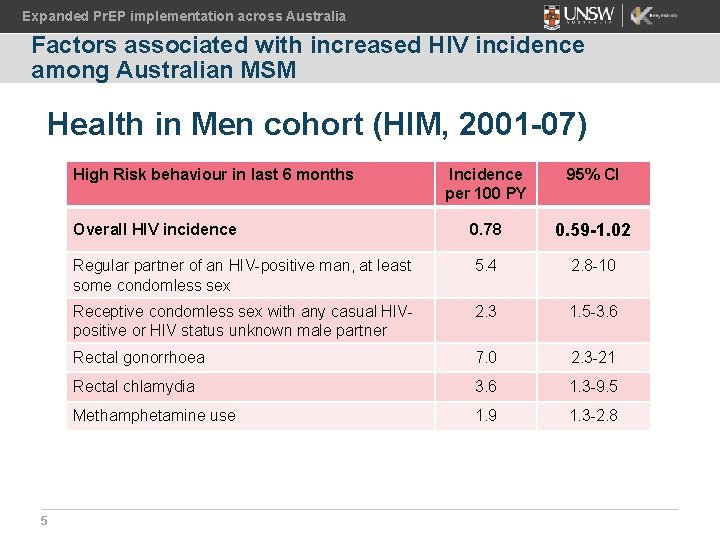
Expanded Pr. EP implementation across Australia Factors associated with increased HIV incidence among Australian MSM Health in Men cohort (HIM, 2001 -07) High Risk behaviour in last 6 months 5 Incidence per 100 PY 95% CI Overall HIV incidence 0. 78 0. 59 -1. 02 Regular partner of an HIV-positive man, at least some condomless sex 5. 4 2. 8 -10 Receptive condomless sex with any casual HIVpositive or HIV status unknown male partner 2. 3 1. 5 -3. 6 Rectal gonorrhoea 7. 0 2. 3 -21 Rectal chlamydia 3. 6 1. 3 -9. 5 Methamphetamine use 1. 9 1. 3 -2. 8

Expanded Pr. EP implementation across Australia The high risk approach to Pr. EP eligibility In the last 3 months: 1. Been the regular sexual partner of an HIV-infected man (not on treatment and/or detectable viral load) and not used condoms consistently 2. Had ≥ 1 episode of receptive condomless anal intercourse (CLAI) with a casual male partner of HIV-positive or unknown status 3. Been diagnosed with rectal gonorrhoea, rectal chlamydia or infectious syphilis 4. Used methamphetamines Recommend prescribing daily Pr. EP Sources: 6 NSW Pr. EP guidelines (2014, updated 2016) Australian Pr. EP guidelines (2015) http: //arv. ashm. org. au/images/Australian_National_Pr. EP_Guidelines. PDF

Expanded Pr. EP implementation across Australia Early Pr. EP demonstration projects in Australia • VICPr. EP (Victoria): 115 participants (2014 - ) • Pr. ELUDE (NSW): 300 participants (2014 - ) • Qprep (Queensland): 50 participants (2016 - ) Lessons learned – Very high levels of adherence – Very high rates of condomless anal intercourse and sexually transmissible infections – Estimated HIV incidence in Pr. ELUDE: 4 per 100 PY – No HIV seroconversions seen in about 500 personyears S Vaccher et al. Pr. ELUDE study. Poster WEPEC 255 7

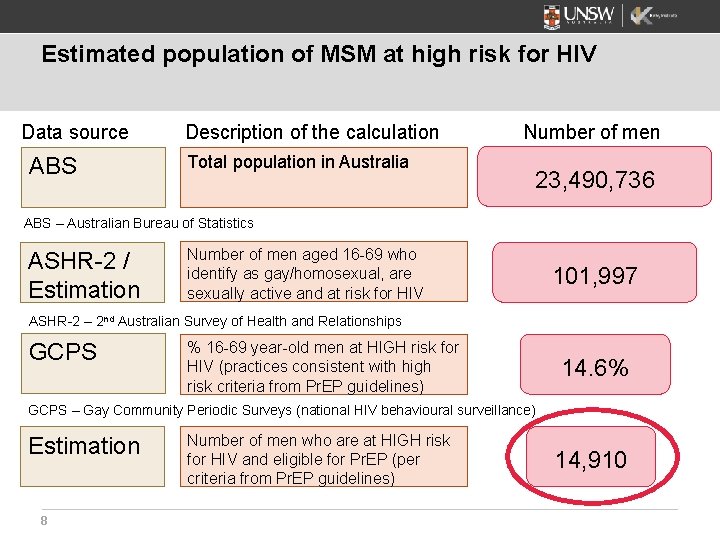
Estimated population of MSM at high risk for HIV Data source ABS Description of the calculation Total population in Australia Number of men 23, 490, 736 ABS – Australian Bureau of Statistics ASHR-2 / Estimation Number of men aged 16 -69 who identify as gay/homosexual, are sexually active and at risk for HIV 101, 997 ASHR-2 – 2 nd Australian Survey of Health and Relationships GCPS % 16 -69 year-old men at HIGH risk for HIV (practices consistent with high risk criteria from Pr. EP guidelines) 14. 6% GCPS – Gay Community Periodic Surveys (national HIV behavioural surveillance) Estimation 8 Number of men who are at HIGH risk for HIV and eligible for Pr. EP (per criteria from Pr. EP guidelines) 14, 910

Expanded Pr. EP implementation across Australia Potential effect of targeted Pr. EP on HIV in NSW • Estimated 3700 high risk MSM are eligible for Pr. EP under current Pr. EP guidelines • Assumptions • • • Expected impact of a Pr. EP access program for 3700 high-risk gay/homosexual men: • 9 HIV incidence is as in Pr. ELUDE study – 4 per 100 py Pr. EP is 86% effective (as in i. PERGAY and PROUD studies) about 150 HIV infections averted in the first 12 months on Pr. EP (~50% of the number HIV diagnoses in NSW in 2014)

Expanded Pr. EP implementation across Australia World AIDS Day 2015 – Health Minister of NSW announces support for EPIC-NSW trial 10

1 March 2016 - EPIC-NSW enrols the first participant I Zablotska et al, 22 July, 11 -1230, Session Room 1 https: //epic-nswstudy. org. au/

“EPIC trial enrols 1000 th participant as access to Pr. EP for HIV prevention expands”

Expanded Pr. EP implementation across Australia 13


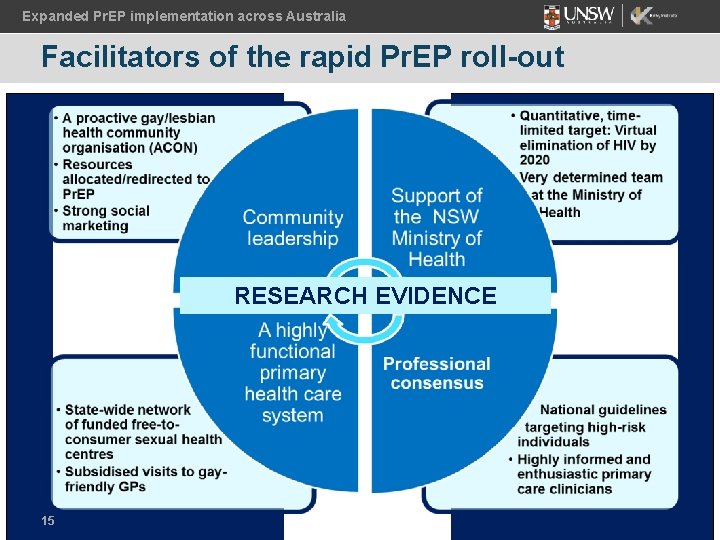
Expanded Pr. EP implementation across Australia Facilitators of the rapid Pr. EP roll-out RESEARCH EVIDENCE 15

Expanded Pr. EP implementation across Australia Research framework – TDF/FTC was not licensed for prevention at the start of the study • Truvada licensed on 6 May 2016 • Generic TDF/FTC not licensed – TDF/FTC is free in the trial context – Evaluation of the study outcomes (HIV, STIs, e. GFRs) 16

Expanded Pr. EP implementation across Australia Further steps in Pr. EP roll-out • Roll-out of Pr. EP implementation trials in Victoria and Queensland 17

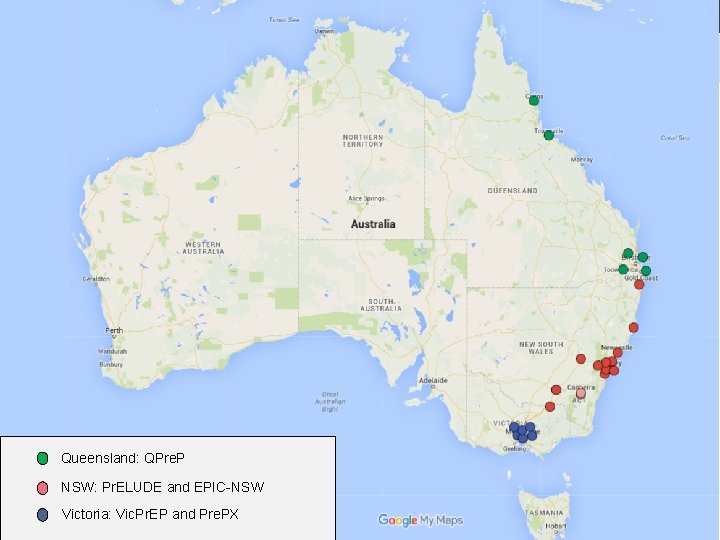
Expanded Pr. EP implementation across Australia Queensland: QPre. P NSW: Pr. ELUDE and EPIC-NSW 18 Victoria: Vic. Pr. EP and Pre. PX

Expanded Pr. EP implementation across Australia Further steps in Pr. EP roll-out • Pr. EP implementation trials in Victoria and Queensland • Other jurisdictions partner with NSW, Victoria and Queensland join large implementation trials • Truvada considered for public subsidy in July 2016 19

Summary Key features of the Australian Pr. EP roll-out: • Time limited target for HIV prevention – to eliminate HIV transmission by 2020 • Evidence-based Pr. EP guidelines • Staged Pr. EP implementation: first step – targeting of highrisk population groups • Strong partnership and community advocacy across HIV sector • Implementation research and ongoing evaluation of Pr. EP rollout

Expanded Pr. EP implementation across Australia The EPIC-NSW team

Expanded Pr. EP implementation across Australia Acknowledgements • Investigator teams of VICPr. EP and QPr. EP studies • State Ministries of Health, particularly the NSW Ministry of Health for funding the implementation trials • Gilead Sciences Inc. for donation of Truvada for Australian trials • Australian Society for HIV Medicine for leadership in policy development • Community organisations in NSW, VIC and Queensland for active support of Pr. EP implementation • The Kirby Institute and UNSW Australia 22