Expanded Access Programs for Drugs and Biologics Richard

Expanded Access Programs for Drugs and Biologics _____________________________ Richard Klein Office of Health and Constituent Affairs Food and Drug Administration February 27, 2014

What is Expanded Access? also called Treatment Use, Compassionate Use • Use of an investigational drug or biologic to treat a patient with a serious disease or condition who does not have comparable or satisfactory alternative therapies to treat the disease or condition. – Intent is clearly treatment - not primarily intended to obtain information about the safety or effectiveness of a drug • Contrast with investigational drug in a clinical trial where the primary intent is research – systematic collection of data with the intent to analyze it to learn about the drug 2
What is Expanded Access? • Patient is not considered part of clinical trial • Minimal data collecting: – Serious/unexpected adverse event – Outcome summary • Not part of study data from trials 3

Expanded Access Programs (EAP) Should Be Option of Last Resort • Approved Drugs: – – Studied and characterized labeled broadest availability 3 rd party reimbursement • Clinical Trials: – Provide necessary data to determine safety & effectiveness • EAP: – Represent opportunity when other options exhausted 4
FDA Published Regulations in 2009 • Subpart I consolidates treatment use into a separate subpart of the IND regulations containing all necessary information • Describes three distinct categories of access – Individual – Intermediate-Size – Treatment IND/protocol • Describes the general criteria applicable to all categories of access, and additional criteria that must be met for each access category • Describes requirements for submission • Describes the safeguards applicable to EAPs (e. g. , informed consent, IRB review, reporting requirements) 5

Requirements for all EAPs 21 CFR 312. 305 • Serious or immediately life threatening illness or condition • No comparable or satisfactory alternative therapy • Potential benefit justifies the potential risks of the treatment, and those risks are not unreasonable in the context of the disease or condition being treated • Providing drug will not interfere with or compromise development for the expanded access use 6

Human Subject Protections Apply to All EAPs Drugs in EAPs are investigational drugs, and they are subject to the following requirements from 21 CFR: – Part 50 - Protection of Human Subjects (informed consent) – Part 56 - Institutional Review Board – Part 312 - including Clinical Holds based on safety and reporting requirements (adverse event reports, annual reports) 7

Requirements for Individual Patient EAPs 21 CFR 312. 310 • Physician must determine probable risk from drug does not exceed that from disease • FDA must determine that the patient cannot obtain access under another type of IND • Procedures for emergency use (where there is not time to make a written IND submission) – FDA may authorize starting access without submission, with very quick turn-around (F/U written submission required within 15 working days of authorization) 8

Requirements for Individual Patient EAPs – Treatment generally limited to one course (though FDA may ok ongoing therapy) – FDA requires written summary report, and may require special monitoring Physician often takes role of sponsor/investigator 9

Intermediate Size Population 21 CFR 312. 315 • Intended for situations where multiple patients with the same condition might benefit from a particular investigational product • No set numerical requirements – meant to be practical and flexible – more than a few, and less than a lot 10

Requirements for Intermediate Size Population 21 CFR 312. 315 • Can be used when a drug is – Being developed (e. g. , patients not eligible for trial) – Not being developed (e. g. , rare disease, cannot recruit for a trial) – Approved (e. g. , drug withdrawn, drug shortage situation- e. g. , foreign version of a U. S. approved drug) 11
Requirements for Intermediate Size Population • Intended for patient populations smaller than intended for Treatment IND (generally up to 100 patients) • FDA can request consolidation when a number of individual requests are received for the same use • Sufficient evidence drug is safe at proposed dose and duration to justify size of exposed population • Preliminary evidence (clinical or plausible pharmacological) of effect • Annual review to determine whether treatment use should be continued and whether a T-IND would be a more appropriate mechanism 12

Requirements for Treatment IND or Protocol 21 CFR 321. 320 • Drug is being investigated in clinical trial designed to support marketing, or trials are complete • Company is actively pursuing marketing approval • Sufficient evidence of safety and effectiveness – Serious disease: evidence from phase 3 or compelling data from phase 2 clinical trials – Immediately life-threatening disease: evidence from phase 3 or phase 2 studies, but could be based on more preliminary clinical evidence 13

FDA Recognizes a Need for Balance • Treatment access must be balanced against the systematic collection of clinical data to characterize safety and effectiveness • Patient autonomy must be balanced against exposure to unreasonable risks and the potential for health fraud, potential exploitation of desperate patients • Individual needs must be balanced against societal needs – Clinical trials are the best mechanism to provide evidence of safety and effectiveness for potential new treatments – FDA approval for marketing is the most efficient means to make safe and effective treatments available to the greatest number of patients. 14

Concern about Trial Enrollment • Early access to investigational therapies could make clinical trials more difficult to perform – E. g. , AZT for HIV, High Dose Chemotherapy + bone marrow transplant for stage IV breast cancer • Clinical trial enrollment and conduct is a factor in consideration of treatment access to experimental drugs • Manufacturing capacity is often limitation in early phases – supply of drug for expanded access could limit supply for trials 15
EAP-Implementing the process • A community responsibility – the patient – the doctor – the sponsor – FDA – IRB 16

EAP-Implementing the process • The patient – Facing difficult medical circumstances and confusing decisions – Need to discover options when others are exhausted – Need to understand accepts potential risks – Patients may face costs that are not reimbursed by health insurers – Navigating uncharted waters that differ significantly from standard health care, e. g. , IRB involvement, informed consent – Patients (and their advising physicians) may have limited information about a drug • confidential commercial information that FDA has access to • may not have access to developing efficacy and/or safety information 17
EAP-Implementing the process • The doctor – Helps initiate the process for the patient – requires commitment to contacting company and filing paperwork • may represent unfamiliar processes for many treating physicians – responsible for ongoing support and monitoring of patient – responsible for adverse event and outcome reporting – Physicians costs of providing access may not be fully compensated – liability issues? 18
EAP-Implementing the process • The sponsor – must be able and willing to provide the product – work with doctor to provide product and monitor product – develop mid-size and large scale program protocols and support program infrastructure • administration • monitoring and reporting responsibilities • IRB review and continuing review 19
EAP-Implementing the process • FDA • IND paperwork • medical records review • quick turn-around time • requires resources – assessment of existing data for safety and evidence of effectiveness – assurance of patient protections (IRB review, informed consent) 20

EAP-Implementing the process • Institutional Review Board (IRB) – outside physician looking for review for their patient – not all IRBs are familiar with expanded access protocols slant of review (intent is treatment, not clinical research) – may miscalculate risk patient is willing to accept – workload and scheduling issues for IRB can delay review – requires entire committee to review (no expedited review procedures) – cost concerns and reimbursement for services 21
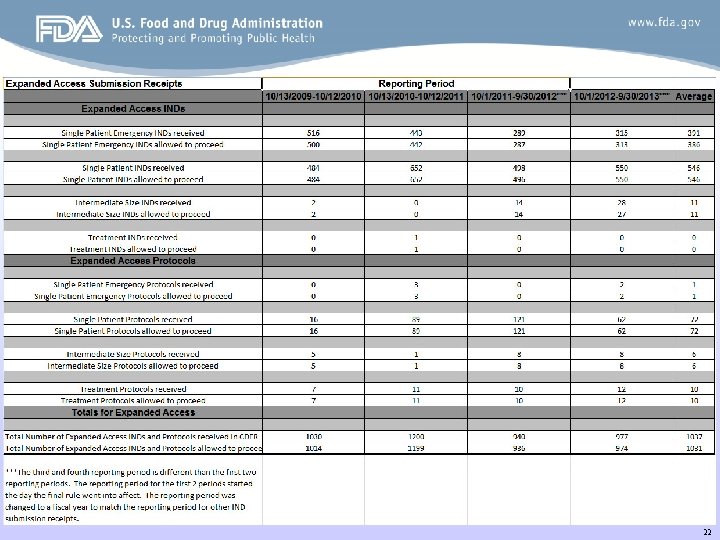
22
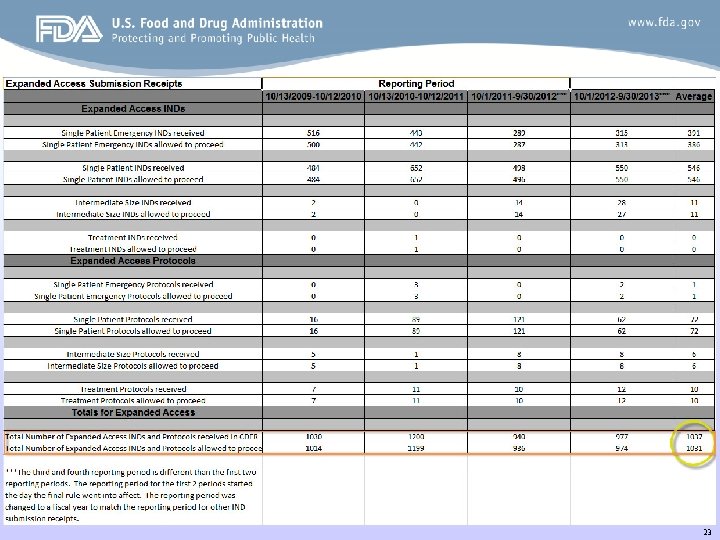
23

How do patients find access programs? – Through their healthcare provider – Internet • Clinical. Trials. gov • Patient organizations • Patient forums – Other patients 24

Take-Away Messages • Patient, physician and sponsor all play integral parts • FDA provides the pathway/mechanism • Sponsor must be able/willing to provide product • Investigational product with more unknowns • Investigator status, IRB, and informed consent 25

Web Resources Access to Investigational Drugs • http: //www. patientnetwork. fda. gov/expanded-access • http: //www. fda. gov/For. Consumers/By. Audience/For. Patient. Advocates/A ccessto. Investigational. Drugs/default. htm Physician Request for an Individual Patient IND under Expanded Access for Non-emergency or Emergency Use • http: //www. fda. gov/Drugs/Development. Approval. Process/How. Drugsar e. Developedand. Approved/Approval. Applications/Investigational. New. Dru g. INDApplication/ucm 107434. htm www. fda. gov, search “expanded access” 26

For Further Information Richard Klein Office of Health and Constituent Affairs (301) 796. 8460 Richard. Klein@fda. hhs. gov www. fda. gov, search “expanded access” 27
- Slides: 27