Exp 7 Empirical Formulas Introduction Compounds are pure



- Slides: 10

Exp 7: Empirical Formulas Introduction Compounds are pure substances – a combination two or more elements that form a new compound Chemical Formula – a combination of symbols of the various elements that make up the compound Formula unit – the smallest collection of atoms that provides information on a compound 1. the identity of the atoms 2. the relative number of each type of atom

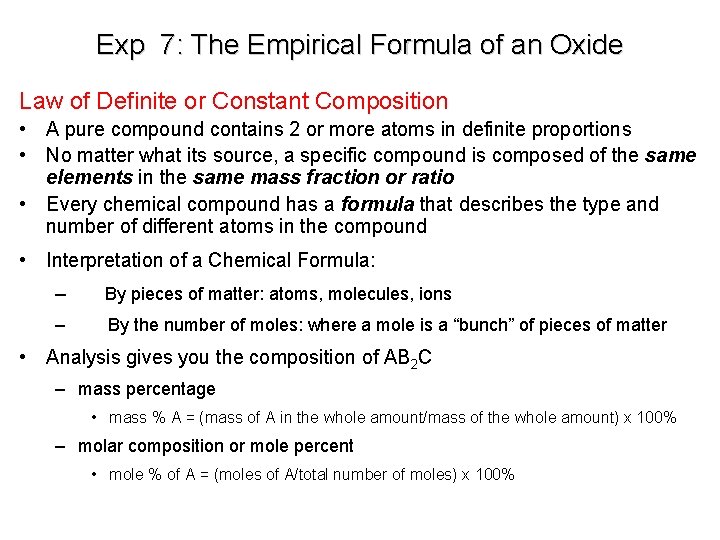
Exp 7: The Empirical Formula of an Oxide Law of Definite or Constant Composition • A pure compound contains 2 or more atoms in definite proportions • No matter what its source, a specific compound is composed of the same elements in the same mass fraction or ratio • Every chemical compound has a formula that describes the type and number of different atoms in the compound • Interpretation of a Chemical Formula: – By pieces of matter: atoms, molecules, ions – By the number of moles: where a mole is a “bunch” of pieces of matter • Analysis gives you the composition of AB 2 C – mass percentage • mass % A = (mass of A in the whole amount/mass of the whole amount) x 100% – molar composition or mole percent • mole % of A = (moles of A/total number of moles) x 100%

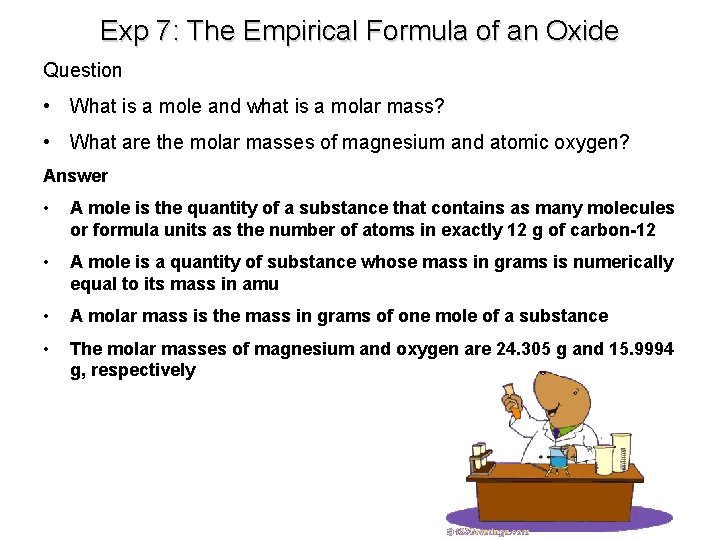
Exp 7: The Empirical Formula of an Oxide Question • What is a mole and what is a molar mass? • What are the molar masses of magnesium and atomic oxygen? Answer • A mole is the quantity of a substance that contains as many molecules or formula units as the number of atoms in exactly 12 g of carbon-12 • A mole is a quantity of substance whose mass in grams is numerically equal to its mass in amu • A molar mass is the mass in grams of one mole of a substance • The molar masses of magnesium and oxygen are 24. 305 g and 15. 9994 g, respectively

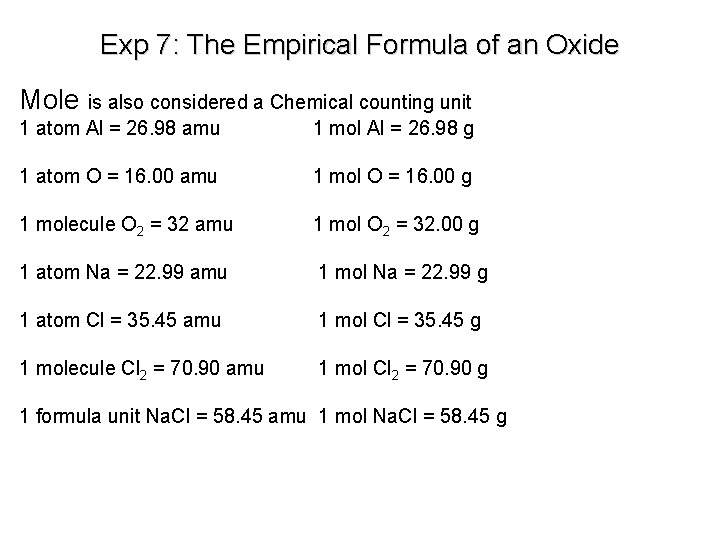
Exp 7: The Empirical Formula of an Oxide Mole is also considered a Chemical counting unit 1 atom Al = 26. 98 amu 1 mol Al = 26. 98 g 1 atom O = 16. 00 amu 1 mol O = 16. 00 g 1 molecule O 2 = 32 amu 1 mol O 2 = 32. 00 g 1 atom Na = 22. 99 amu 1 mol Na = 22. 99 g 1 atom Cl = 35. 45 amu 1 mol Cl = 35. 45 g 1 molecule Cl 2 = 70. 90 amu 1 mol Cl 2 = 70. 90 g 1 formula unit Na. Cl = 58. 45 amu 1 mol Na. Cl = 58. 45 g

Exp 7: The Empirical Formula of an Oxide NOTES for the EXPERIMENT Purpose • • Observe the reaction of magnesium with oxygen Determine the empirical formula of the product, magnesium oxide Background • Oxygen (O 2) is very reactive when heated • Many elements react with oxygen, forming an “oxide” • • Nitrogen (N 2) is very unreactive, even at high temperature Only very active metals react with N 2, forming a “nitride” Water (H 2 O) and nitrides react to form “hydroxides” (compounds of a metal and a hydroxide, OH) and ammonia, NH 3. Heating the hydroxide converts it to an oxide and water vapor

Exp 7: The Empirical Formula of an Oxide Background • Final product is magnesium oxide 1. Primary reaction Reaction between Mg and O 2 Mg. O 2. Secondary reaction - Reaction between Mg and N 2 Mg 3 N 2 - Mg 3 N 2 and water (H 2 O) form Mg(OH)2 - Heating Mg(OH)2 results in formation of Mg. O and H 2 O 3. All Mg is now converted to Mg. O Mass of O 2 that reacted with Mg can be determined from the original mass of Mg and the mass of the final product, Mg. O 4. Laws of conservation of mass and number of moles are used to calculate this amount

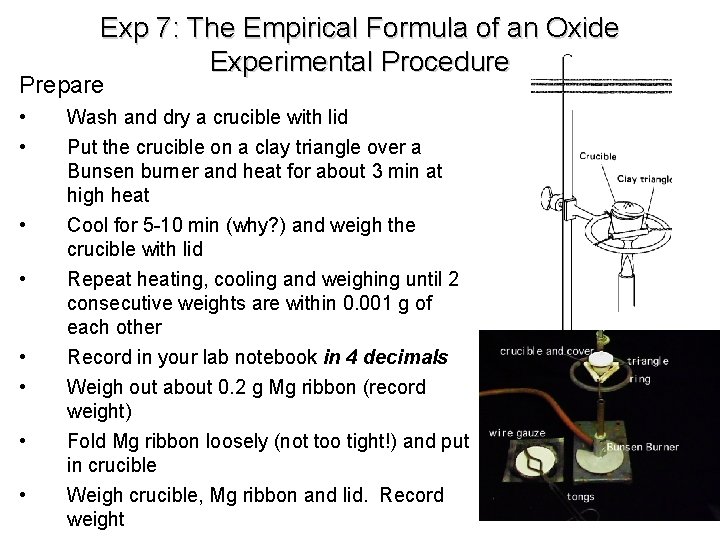
Exp 7: The Empirical Formula of an Oxide Experimental Procedure Prepare • • Wash and dry a crucible with lid Put the crucible on a clay triangle over a Bunsen burner and heat for about 3 min at high heat Cool for 5 -10 min (why? ) and weigh the crucible with lid Repeat heating, cooling and weighing until 2 consecutive weights are within 0. 001 g of each other Record in your lab notebook in 4 decimals Weigh out about 0. 2 g Mg ribbon (record weight) Fold Mg ribbon loosely (not too tight!) and put in crucible Weigh crucible, Mg ribbon and lid. Record weight

Exp 7: The Empirical Formula of an Oxide Experimental Procedure Heating • • Put crucible, Mg ribbon and lid on clay triangle. Cover crucible with lid. “Brush” bottom for 2 -3 min with hot flame Put burner under crucible and heat for 3 more min in the hottest part of the flame Lift lid slightly with tongs to allow air to enter – – • Repeat approximately every 3 -5 min until no metal is visible anymore – – • Don’t open too far, because Mg will catch fire Metal should glow bright-red all is converted to magnesium oxide powder no glowing is visible anymore Allow crucible to cool

Exp 7: The Empirical Formula of an Oxide Experimental Procedure Analysis • • • When the crucible has cooled down to the point where it is close to room temperature – you feel no heat when you bring your finger within ½ in of the crucible Weigh the crucible + content + lid. Record the weight Heat again for 3 min Cool crucible and obtain weight; record weight in 4 decimals Repeat until weight is constant – 2 consecutive weightings within 0. 001 g of each other

Exp 7: The Empirical Formula of an Oxide Chemicals Post-Lab Assignment due next week • Fill in the Report Sheet (p. 115) – Omit 9, 10, 11 – Show all your calculations on a separate sheet of paper. • Answer questions 1 through 4 on Laboratory questions following your calculations on the sheet of paper • Critical Thinking (no need to write): Think carefully about the precision of the masses that you determined on the electronic balance. How many significant figures are justified in your answer? Also due: Pre-lab for next lab