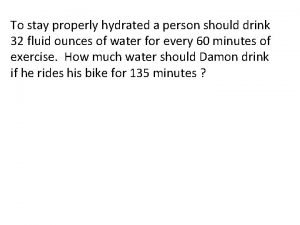Exp 5 Percent Water in a Hydrated Salt






- Slides: 10

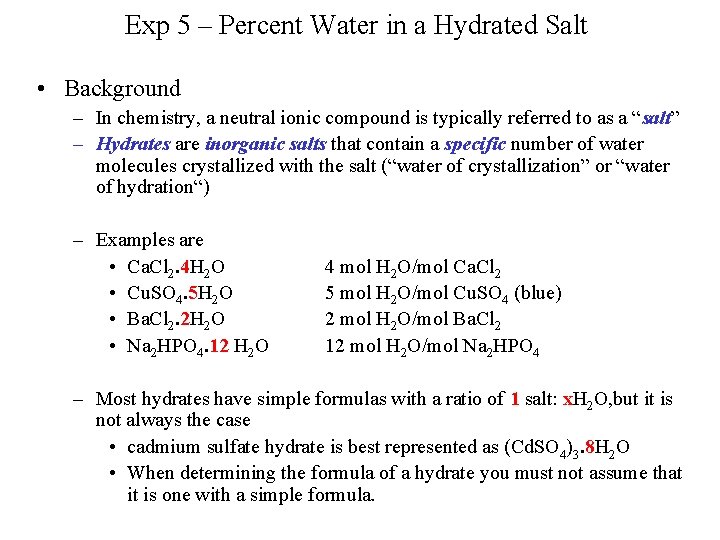
Exp 5 – Percent Water in a Hydrated Salt • Background – In chemistry, a neutral ionic compound is typically referred to as a “salt” – Hydrates are inorganic salts that contain a specific number of water molecules crystallized with the salt (“water of crystallization” or “water of hydration“) – Examples are • Ca. Cl 2. 4 H 2 O • Cu. SO 4. 5 H 2 O • Ba. Cl 2. 2 H 2 O • Na 2 HPO 4. 12 H 2 O 4 mol H 2 O/mol Ca. Cl 2 5 mol H 2 O/mol Cu. SO 4 (blue) 2 mol H 2 O/mol Ba. Cl 2 12 mol H 2 O/mol Na 2 HPO 4 – Most hydrates have simple formulas with a ratio of 1 salt: x. H 2 O, but it is not always the case • cadmium sulfate hydrate is best represented as (Cd. SO 4)3. 8 H 2 O • When determining the formula of a hydrate you must not assume that it is one with a simple formula.

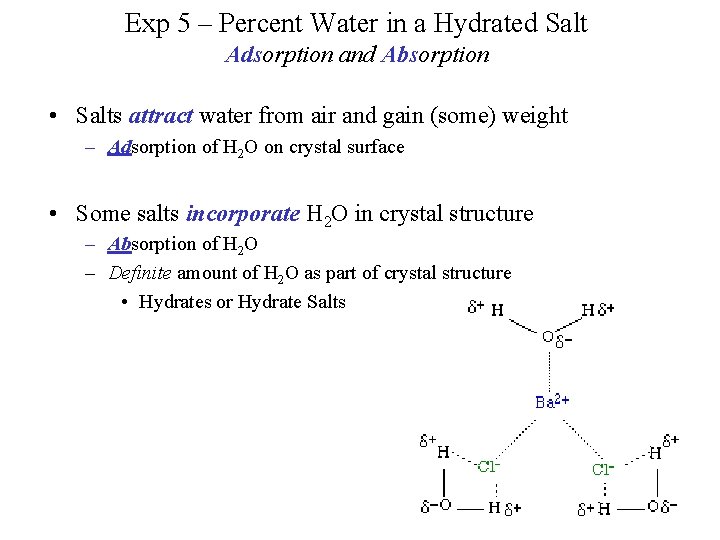
Exp 5 – Percent Water in a Hydrated Salt Adsorption and Absorption • Salts attract water from air and gain (some) weight – Adsorption of H 2 O on crystal surface • Some salts incorporate H 2 O in crystal structure – Absorption of H 2 O – Definite amount of H 2 O as part of crystal structure • Hydrates or Hydrate Salts

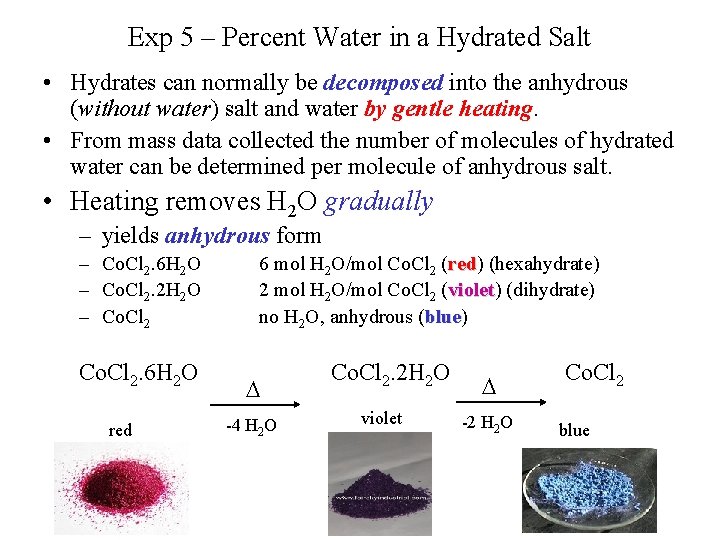
Exp 5 – Percent Water in a Hydrated Salt • Hydrates can normally be decomposed into the anhydrous (without water) salt and water by gentle heating. • From mass data collected the number of molecules of hydrated water can be determined per molecule of anhydrous salt. • Heating removes H 2 O gradually – yields anhydrous form – Co. Cl 2. 6 H 2 O – Co. Cl 2. 2 H 2 O – Co. Cl 2. 6 H 2 O red 6 mol H 2 O/mol Co. Cl 2 (red) red (hexahydrate) 2 mol H 2 O/mol Co. Cl 2 (violet) violet (dihydrate) no H 2 O, anhydrous (blue) blue D -4 H 2 O Co. Cl 2. 2 H 2 O violet D -2 H 2 O Co. Cl 2 blue

Exp 5 – Percent Water in a Hydrated Salt • Some salts spontaneously lose water molecules to the atmosphere: efflorescent • Some salts spontaneously absorb water from the atmosphere: deliquescent • Determinations carried out by measurements of mass throughout the experiment are referred to as gravimetric analysis • Thus, the determination of % by mass of water in a hydrated salt uses gravimetric analysis.

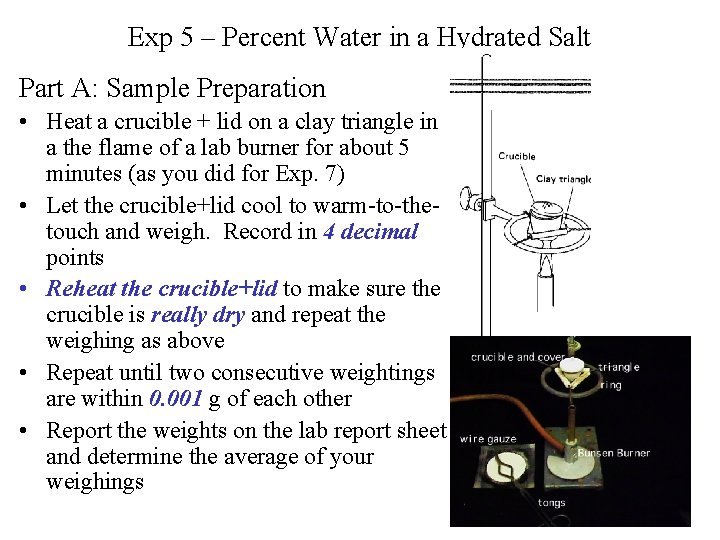
Exp 5 – Percent Water in a Hydrated Salt Part A: Sample Preparation • Heat a crucible + lid on a clay triangle in a the flame of a lab burner for about 5 minutes (as you did for Exp. 7) • Let the crucible+lid cool to warm-to-thetouch and weigh. Record in 4 decimal points • Reheat the crucible+lid to make sure the crucible is really dry and repeat the weighing as above • Repeat until two consecutive weightings are within 0. 001 g of each other • Report the weights on the lab report sheet and determine the average of your weighings

Exp 5 – Percent Water in a Hydrated Salt Part A (continued): 1. Average mass of crucible+lid 2. Add about 2. 0 – 2. 5 g sample into the fired cool crucible 3. Weigh crucible + lid + hydrate sample (0. 0001 g precision) 4. Mass of hydrate sample = (crucible + lid + hydrate sample ) – (crucible+lid) Part B: Thermal decomposition product of sample 1. Heat the crucible gently for 2 -3 min with lid slightly ajar Continue heating at full flame for 10 min Cool crucible to “warm to touch” and weigh 2. Repeat heating for 2 min, and cool crucible (“warm to touch”) Weigh crucible + lid + residue (0. 0001 g precision) Record as Final mass if the difference from previous weighing is not more than + or - 0. 010 g Mass(g)

Exp 5 – Percent Water in a Hydrated Salt Calculations: • #4. Percent by Mass of volatile water % by mass= mass water x 100% mass hydrated salt • #6. Standard Deviation* of % H 2 O: • Measures the spread of your results (how far they are from each other) • #7. Relative Standard Deviation* (%RSD): • Measures the spread of the individual results compared to the average result

Exp 5 – Additional Information • Why is it important to apply heat only as instructed? The salt itself could decompose into other compounds • Can correct % calculations still be obtained? Yes but you must know the formulas of the possible decomposition products and do extra calculations _

CORRECTION: • USE an amount of sample ~ 1. 5 to 2. 0 g for a narrow crucible • USE 2. 0 -2. 5 g for a wider-mouth crucible

Next week Due Monday July 1, 2013 Exp 5 done today • Report sheets p. 83 -84 • Questions #1 -7 on p. 84 Next week’s Exp 2 p. 53: • • Prelab assignment: answer Read and understand: – – – Goal of Experimental Procedures Safety Precautions for this lab
 Percent by mass of volatile water in hydrated salt
Percent by mass of volatile water in hydrated salt Water and water and water water
Water and water and water water Fresh water meets salt water
Fresh water meets salt water To stay properly hydrated a person
To stay properly hydrated a person Hydrated lime uses
Hydrated lime uses Hydrated copper carbonate
Hydrated copper carbonate Cobalt(ii) chloride hydrated and dehydrated
Cobalt(ii) chloride hydrated and dehydrated Valid percent
Valid percent Natural logarithm in matlab
Natural logarithm in matlab Exp 13
Exp 13 Energy band diagram of pnp transistor
Energy band diagram of pnp transistor