Exp 2 Identification of a Compound Physical Properties



- Slides: 9

Exp 2: Identification of a Compound: Physical Properties Introduction • Chemistry is based on the relation between experimental observations and theory – – – Experiments lead to theories lead to other experiments Accurate, precise and complete observations are the basis for successful experiments Without good observations there is no basis for developing a coherent theory Purpose • Identify an unknown compound based on properties of known compounds • Practice proper and accurate observations and focus on the conclusions that can be drawn from those observation • Correct and complete observations are the only tools that you need for this experiment

Exp 2: Identification of a Compound Successful qualitative analysis “. . . when you have excluded the impossible, whatever remains must be the truth. ” (Sherlock Holmes)

Exp 2: Identification of a Compound Experimental Concept • Determine the identity of an unknown compound: – observe chemical reaction of a series of solutions of known solid compounds – observe the reactions of an unknown compound and compare to the known compounds • Known compounds – – – sodium chloride, Na. Cl sodium carbonate, Na 2 CO 3 magnesium sulfate, Mg. SO 4 ammonium chloride, NH 4 Cl water, H 2 O

Exp 2: Identification of a Compound Experimental Concept (cont’d) • Test compounds for – reaction of solutions with silver nitrate reagent, Ag. NO 3 – reaction of solutions with sodium hydroxide reagent, Na. OH – reaction of solutions with hydrochloric acid reagent, HCl • Observe Evidence of a Reaction (chemical change) – change of color in a solution – change in odor of a solution – evolution of a gas • bubbles forming in the solution (not just a few, but a lot) – appearance or disappearance of a precipitate (insoluble material) • solids can be so fine that they are sometimes hard to see • a precipitate will eventually settle at the bottom of a test tube

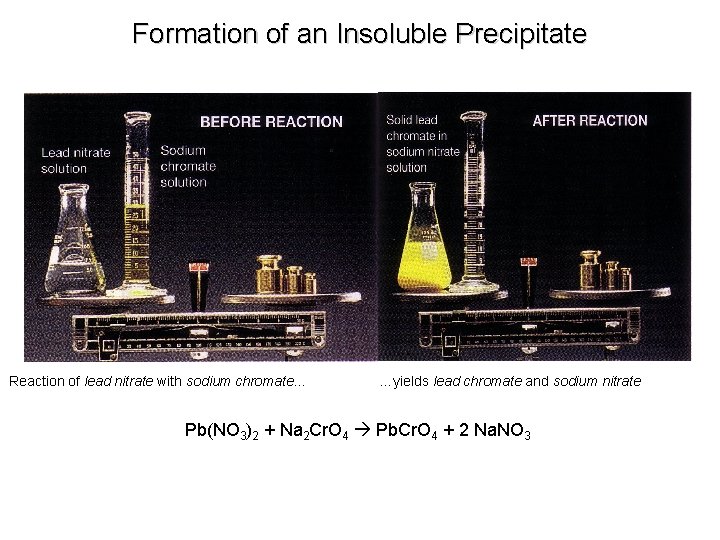
Formation of an Insoluble Precipitate Reaction of lead nitrate with sodium chromate… …yields lead chromate and sodium nitrate Pb(NO 3)2 + Na 2 Cr. O 4 Pb. Cr. O 4 + 2 Na. NO 3

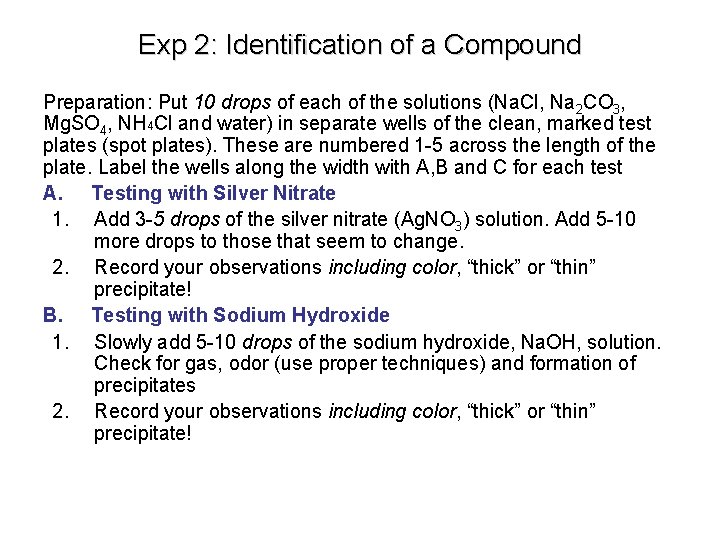
Exp 2: Identification of a Compound Preparation: Put 10 drops of each of the solutions (Na. Cl, Na 2 CO 3, Mg. SO 4, NH 4 Cl and water) in separate wells of the clean, marked test plates (spot plates). These are numbered 1 -5 across the length of the plate. Label the wells along the width with A, B and C for each test A. Testing with Silver Nitrate 1. Add 3 -5 drops of the silver nitrate (Ag. NO 3) solution. Add 5 -10 more drops to those that seem to change. 2. Record your observations including color, “thick” or “thin” precipitate! B. Testing with Sodium Hydroxide 1. Slowly add 5 -10 drops of the sodium hydroxide, Na. OH, solution. Check for gas, odor (use proper techniques) and formation of precipitates 2. Record your observations including color, “thick” or “thin” precipitate!

Exp 2: Identification of a Compound D. Testing with Hydrochloric Acid 1. Slowly add 5 -10 drops of the hydrochloric acid reagent, HCl, to each solution. Check for gas, odor (use proper techniques). 2. Observe closely; Record your observations including color, odor if any Testing the unknown solution: 1. Place 10 drops of the unknown solution in three wells of column 6 2. Test with the reagents, using the same proportions as with the known solutions. Compare results and identify your unknown. Clean up: 1. Dispose solutions in the liquid waste container in the fume hood 2. Rinse the plates with tap water and DI water

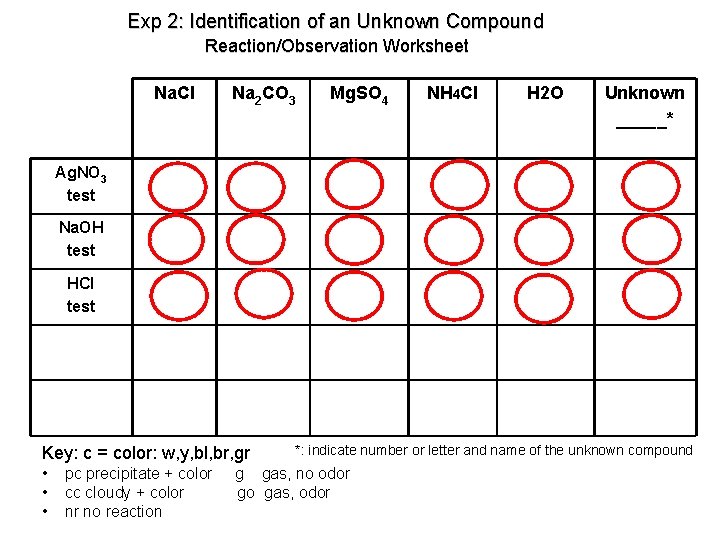
Exp 2: Identification of an Unknown Compound Reaction/Observation Worksheet Na. Cl Na 2 CO 3 Mg. SO 4 NH 4 Cl H 2 O Unknown _____* Ag. NO 3 test Na. OH test HCl test Key: c = color: w, y, bl, br, gr • • • pc precipitate + color cc cloudy + color nr no reaction *: indicate number or letter and name of the unknown compound g gas, no odor go gas, odor

Exp 2: Identification of a Compound Next week § SPRING BREAK: Study for Lecture Quiz Monday § Tuesday June 9: Turn in Pre-lab for Exp 10: Vinegar Analysis Ø Read procedure and safety precautions