Exp 19 A OxidationReduction Reactions OxidationReduction Redox reactions



- Slides: 19

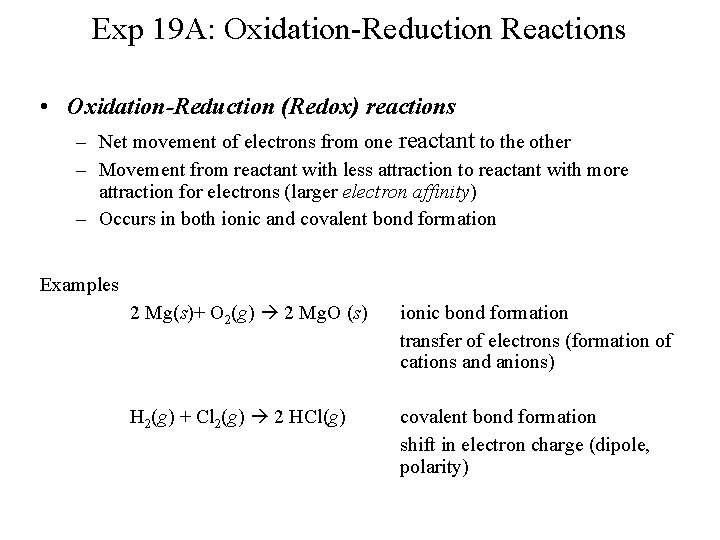
Exp 19 A: Oxidation-Reduction Reactions • Oxidation-Reduction (Redox) reactions – Net movement of electrons from one reactant to the other – Movement from reactant with less attraction to reactant with more attraction for electrons (larger electron affinity) – Occurs in both ionic and covalent bond formation Examples 2 Mg(s)+ O 2(g) 2 Mg. O (s) ionic bond formation transfer of electrons (formation of cations and anions) H 2(g) + Cl 2(g) 2 HCl(g) covalent bond formation shift in electron charge (dipole, polarity)

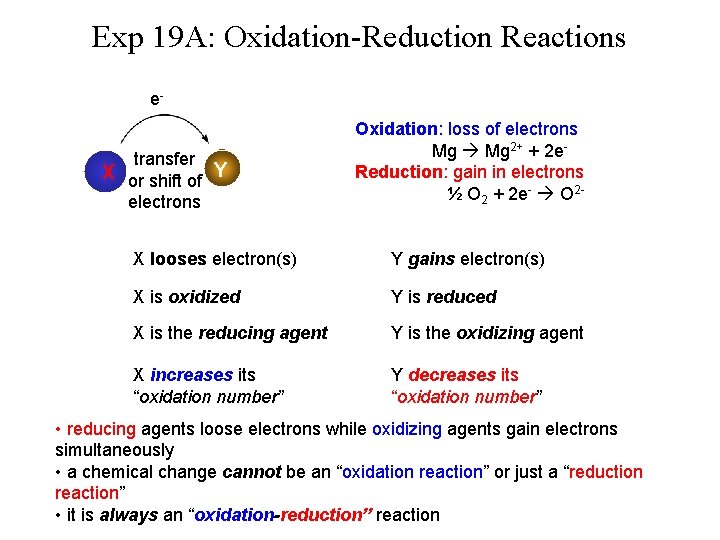
Exp 19 A: Oxidation-Reduction Reactions etransfer X or shift of Y electrons Oxidation: loss of electrons Mg 2+ + 2 e. Reduction: gain in electrons ½ O 2 + 2 e- O 2 - X looses electron(s) Y gains electron(s) X is oxidized Y is reduced X is the reducing agent Y is the oxidizing agent X increases its “oxidation number” Y decreases its “oxidation number” • reducing agents loose electrons while oxidizing agents gain electrons simultaneously • a chemical change cannot be an “oxidation reaction” or just a “reduction reaction” • it is always an “oxidation-reduction” reaction

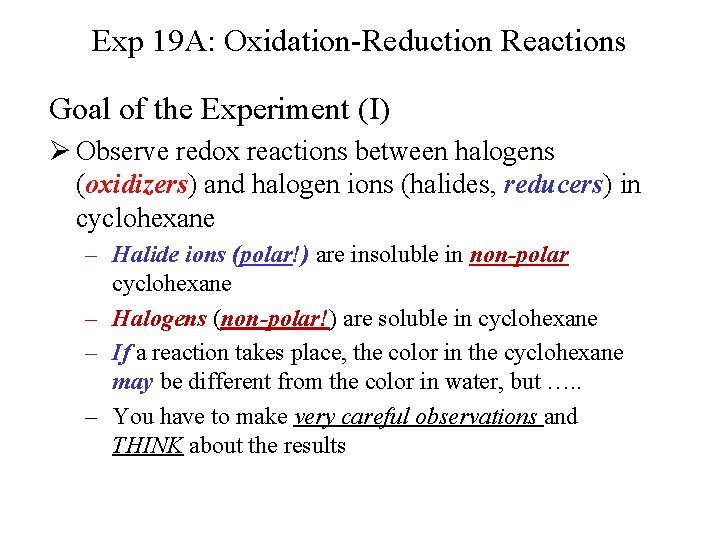
Exp 19 A: Oxidation-Reduction Reactions Goal of the Experiment (I) Ø Observe redox reactions between halogens (oxidizers) and halogen ions (halides, reducers) in cyclohexane – Halide ions (polar!) are insoluble in non-polar cyclohexane – Halogens (non-polar!) are soluble in cyclohexane – If a reaction takes place, the color in the cyclohexane may be different from the color in water, but …. . – You have to make very careful observations and THINK about the results

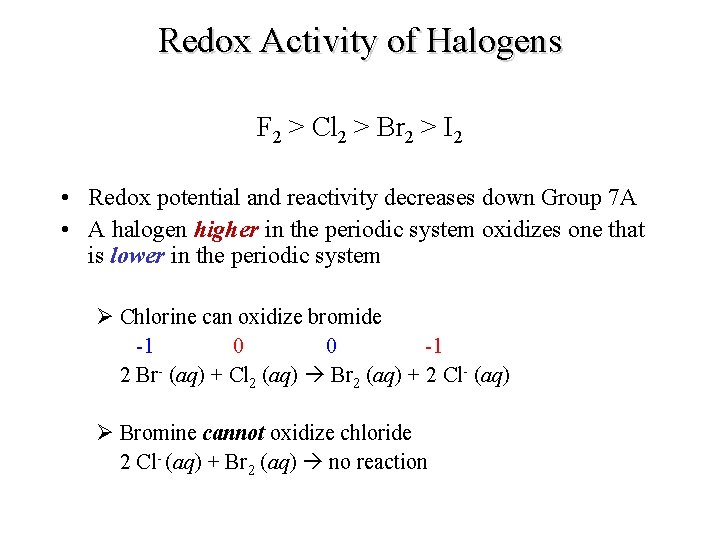
Redox Activity of Halogens F 2 > Cl 2 > Br 2 > I 2 • Redox potential and reactivity decreases down Group 7 A • A halogen higher in the periodic system oxidizes one that is lower in the periodic system Ø Chlorine can oxidize bromide -1 0 0 -1 2 Br- (aq) + Cl 2 (aq) Br 2 (aq) + 2 Cl- (aq) Ø Bromine cannot oxidize chloride 2 Cl- (aq) + Br 2 (aq) no reaction

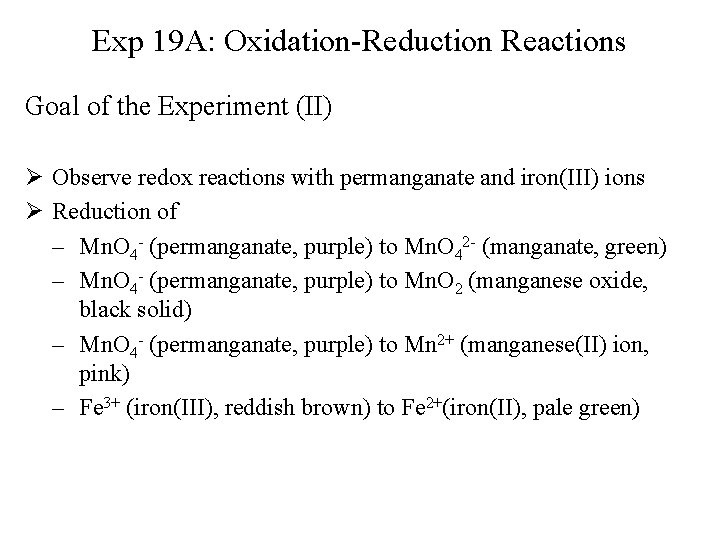
Exp 19 A: Oxidation-Reduction Reactions Goal of the Experiment (II) Ø Observe redox reactions with permanganate and iron(III) ions Ø Reduction of – Mn. O 4 - (permanganate, purple) to Mn. O 42 - (manganate, green) – Mn. O 4 - (permanganate, purple) to Mn. O 2 (manganese oxide, black solid) – Mn. O 4 - (permanganate, purple) to Mn 2+ (manganese(II) ion, pink) – Fe 3+ (iron(III), reddish brown) to Fe 2+(iron(II), pale green)

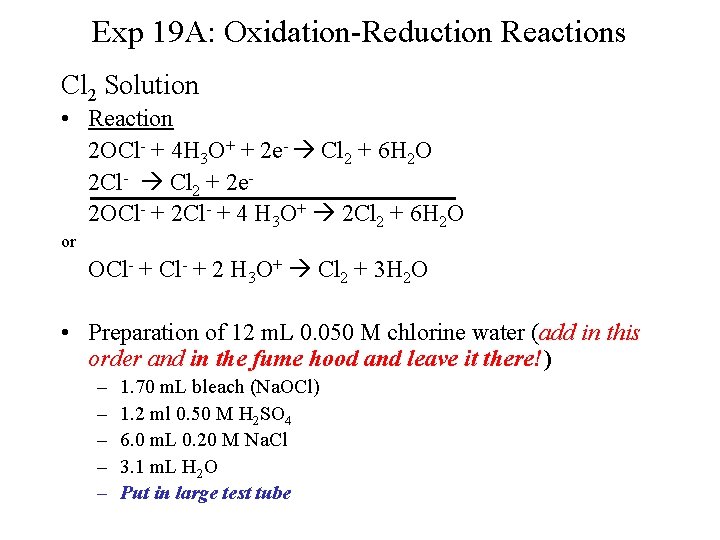
Exp 19 A: Oxidation-Reduction Reactions Cl 2 Solution • Reaction 2 OCl- + 4 H 3 O+ + 2 e- Cl 2 + 6 H 2 O 2 Cl- Cl 2 + 2 e 2 OCl- + 2 Cl- + 4 H 3 O+ 2 Cl 2 + 6 H 2 O or OCl- + 2 H 3 O+ Cl 2 + 3 H 2 O • Preparation of 12 m. L 0. 050 M chlorine water (add in this order and in the fume hood and leave it there!) – – – 1. 70 m. L bleach (Na. OCl) 1. 2 ml 0. 50 M H 2 SO 4 6. 0 m. L 0. 20 M Na. Cl 3. 1 m. L H 2 O Put in large test tube

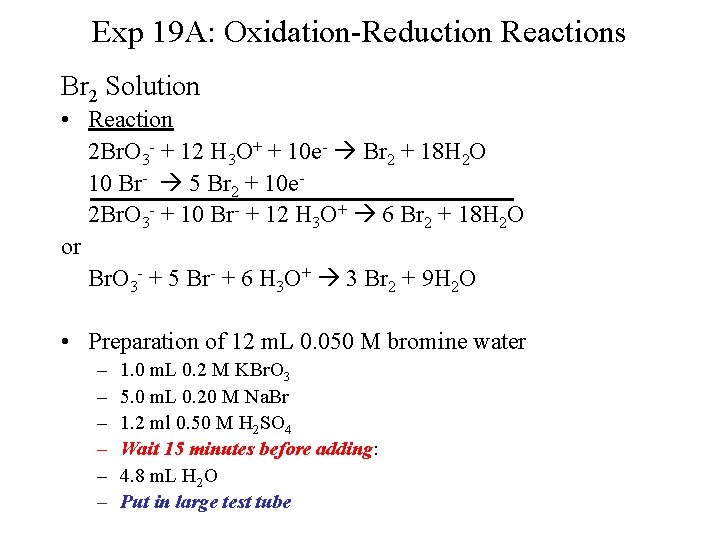
Exp 19 A: Oxidation-Reduction Reactions Br 2 Solution • Reaction 2 Br. O 3 - + 12 H 3 O+ + 10 e- Br 2 + 18 H 2 O 10 Br- 5 Br 2 + 10 e 2 Br. O 3 - + 10 Br- + 12 H 3 O+ 6 Br 2 + 18 H 2 O or Br. O 3 - + 5 Br- + 6 H 3 O+ 3 Br 2 + 9 H 2 O • Preparation of 12 m. L 0. 050 M bromine water – – – 1. 0 m. L 0. 2 M KBr. O 3 5. 0 m. L 0. 20 M Na. Br 1. 2 ml 0. 50 M H 2 SO 4 Wait 15 minutes before adding: 4. 8 m. L H 2 O Put in large test tube

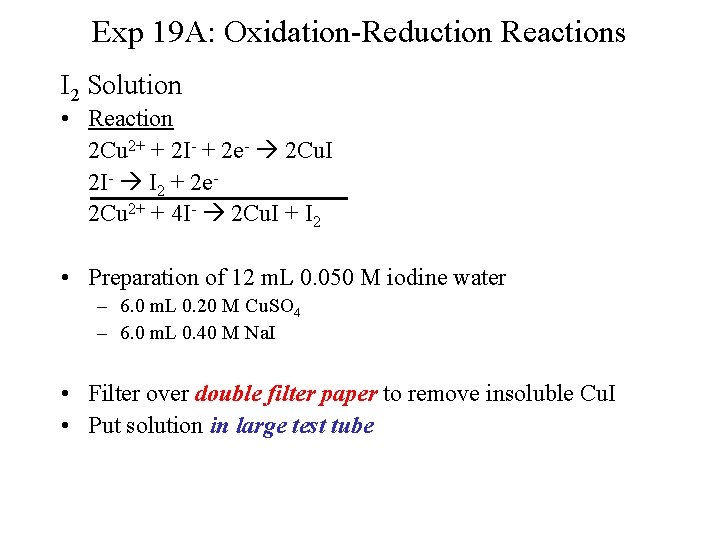
Exp 19 A: Oxidation-Reduction Reactions I 2 Solution • Reaction 2 Cu 2+ + 2 I- + 2 e- 2 Cu. I 2 I- I 2 + 2 e 2 Cu 2+ + 4 I- 2 Cu. I + I 2 • Preparation of 12 m. L 0. 050 M iodine water – 6. 0 m. L 0. 20 M Cu. SO 4 – 6. 0 m. L 0. 40 M Na. I • Filter over double filter paper to remove insoluble Cu. I • Put solution in large test tube

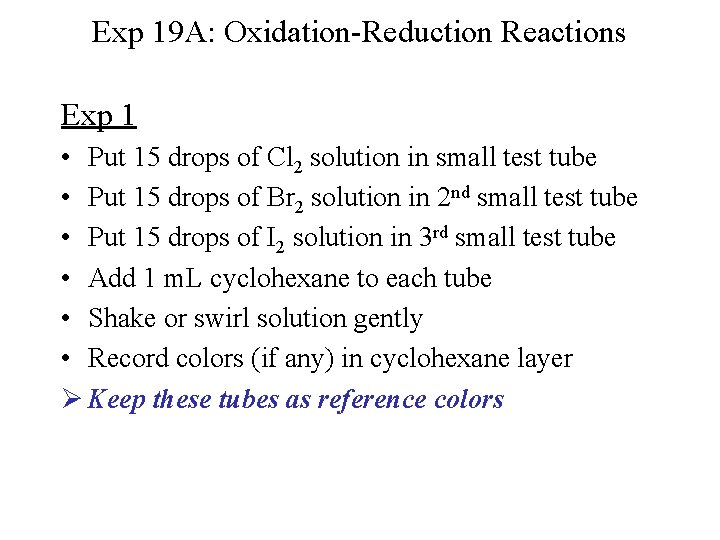
Exp 19 A: Oxidation-Reduction Reactions Exp 1 • Put 15 drops of Cl 2 solution in small test tube • Put 15 drops of Br 2 solution in 2 nd small test tube • Put 15 drops of I 2 solution in 3 rd small test tube • Add 1 m. L cyclohexane to each tube • Shake or swirl solution gently • Record colors (if any) in cyclohexane layer Ø Keep these tubes as reference colors

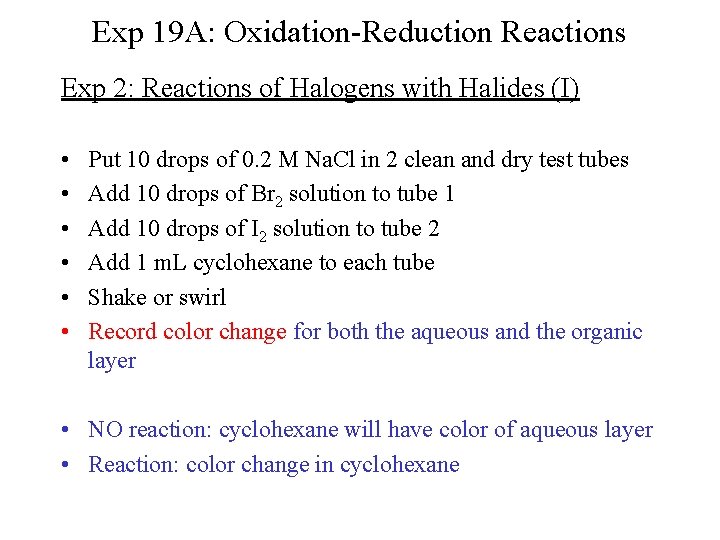
Exp 19 A: Oxidation-Reduction Reactions Exp 2: Reactions of Halogens with Halides (I) • • • Put 10 drops of 0. 2 M Na. Cl in 2 clean and dry test tubes Add 10 drops of Br 2 solution to tube 1 Add 10 drops of I 2 solution to tube 2 Add 1 m. L cyclohexane to each tube Shake or swirl Record color change for both the aqueous and the organic layer • NO reaction: cyclohexane will have color of aqueous layer • Reaction: color change in cyclohexane

Exp 19 A: Oxidation-Reduction Reactions Exp 2: Reactions of Halogens with Halides (II) • • Clean test tubes Put 10 drops of 0. 2 M Na. Br in 2 clean and dry test tubes Add 10 drops of Cl 2 solution to tube 1 Add 10 drops of I 2 solution to tube 2 Add 1 m. L cyclohexane to each tube Shake or swirl Record color change for both the aqueous and the organic layer

Exp 19 A: Oxidation-Reduction Reactions Exp 2: Reactions of Halogens with Halides (III) • • Clean test tubes Put 5 drops of 0. 4 M Na. I in 2 clean and dry test tubes Add 10 drops of Cl 2 solution to tube 1 Add 10 drops of Br 2 solution to tube 2 Add 1 m. L cyclohexane to each tube Shake or swirl Record color change for both the aqueous and the organic layer

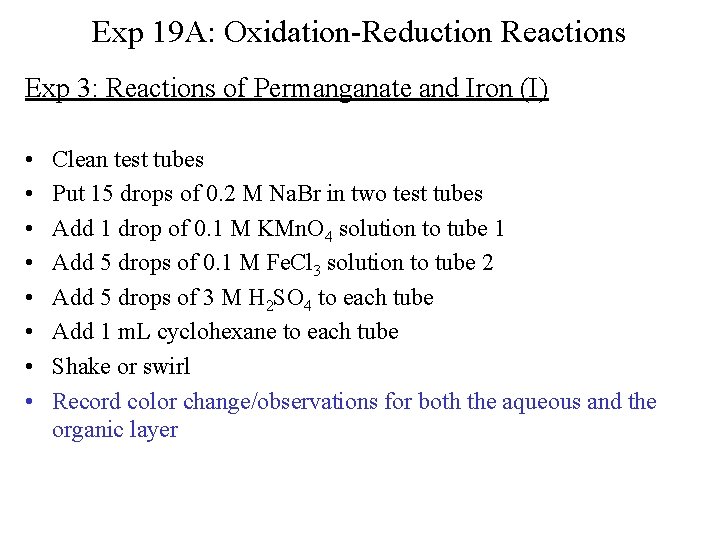
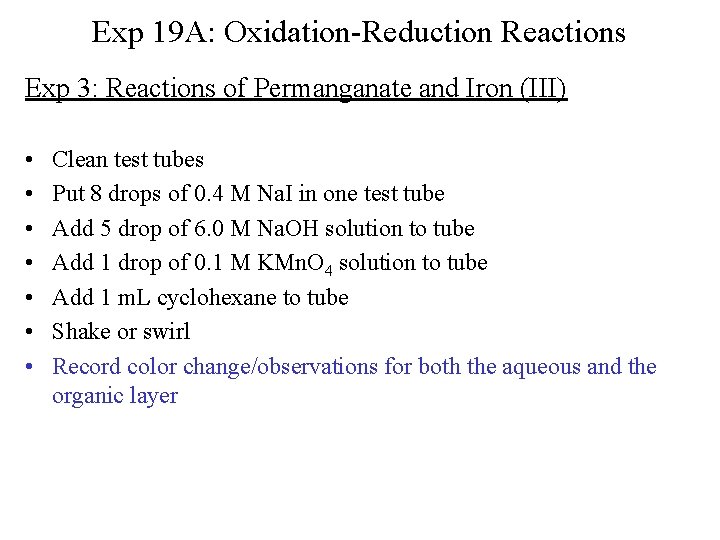
Exp 19 A: Oxidation-Reduction Reactions Exp 3: Reactions of Permanganate and Iron (I) • • Clean test tubes Put 15 drops of 0. 2 M Na. Br in two test tubes Add 1 drop of 0. 1 M KMn. O 4 solution to tube 1 Add 5 drops of 0. 1 M Fe. Cl 3 solution to tube 2 Add 5 drops of 3 M H 2 SO 4 to each tube Add 1 m. L cyclohexane to each tube Shake or swirl Record color change/observations for both the aqueous and the organic layer

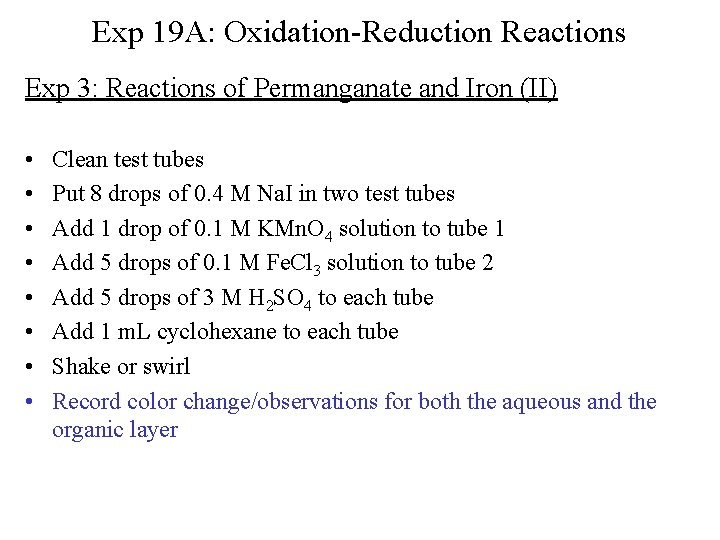
Exp 19 A: Oxidation-Reduction Reactions Exp 3: Reactions of Permanganate and Iron (II) • • Clean test tubes Put 8 drops of 0. 4 M Na. I in two test tubes Add 1 drop of 0. 1 M KMn. O 4 solution to tube 1 Add 5 drops of 0. 1 M Fe. Cl 3 solution to tube 2 Add 5 drops of 3 M H 2 SO 4 to each tube Add 1 m. L cyclohexane to each tube Shake or swirl Record color change/observations for both the aqueous and the organic layer

Exp 19 A: Oxidation-Reduction Reactions Exp 3: Reactions of Permanganate and Iron (III) • • Clean test tubes Put 8 drops of 0. 4 M Na. I in one test tube Add 5 drop of 6. 0 M Na. OH solution to tube Add 1 drop of 0. 1 M KMn. O 4 solution to tube Add 1 m. L cyclohexane to tube Shake or swirl Record color change/observations for both the aqueous and the organic layer

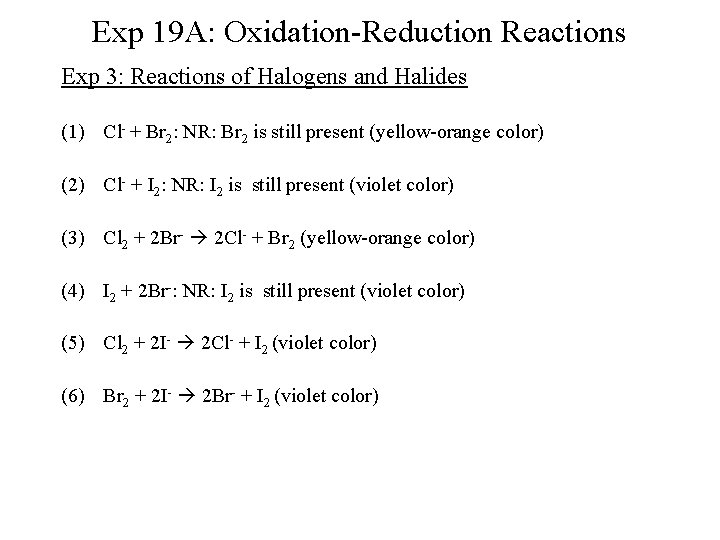
Exp 19 A: Oxidation-Reduction Reactions Exp 3: Reactions of Halogens and Halides (1) Cl- + Br 2: NR: Br 2 is still present (yellow-orange color) (2) Cl- + I 2: NR: I 2 is still present (violet color) (3) Cl 2 + 2 Br- 2 Cl- + Br 2 (yellow-orange color) (4) I 2 + 2 Br-: NR: I 2 is still present (violet color) (5) Cl 2 + 2 I- 2 Cl- + I 2 (violet color) (6) Br 2 + 2 I- 2 Br- + I 2 (violet color)

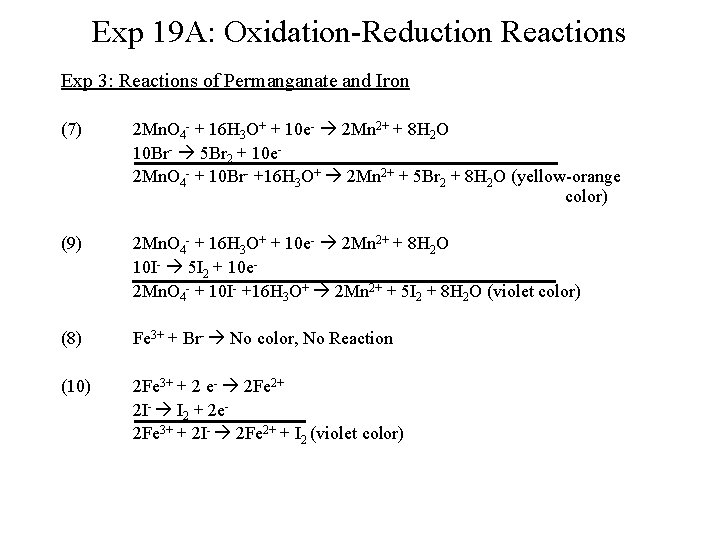
Exp 19 A: Oxidation-Reduction Reactions Exp 3: Reactions of Permanganate and Iron (7) 2 Mn. O 4 - + 16 H 3 O+ + 10 e- 2 Mn 2+ + 8 H 2 O 10 Br- 5 Br 2 + 10 e 2 Mn. O 4 - + 10 Br- +16 H 3 O+ 2 Mn 2+ + 5 Br 2 + 8 H 2 O (yellow-orange color) (9) 2 Mn. O 4 - + 16 H 3 O+ + 10 e- 2 Mn 2+ + 8 H 2 O 10 I- 5 I 2 + 10 e 2 Mn. O 4 - + 10 I- +16 H 3 O+ 2 Mn 2+ + 5 I 2 + 8 H 2 O (violet color) (8) Fe 3+ + Br- No color, No Reaction (10) 2 Fe 3+ + 2 e- 2 Fe 2+ 2 I- I 2 + 2 e 2 Fe 3+ + 2 I- 2 Fe 2+ + I 2 (violet color)

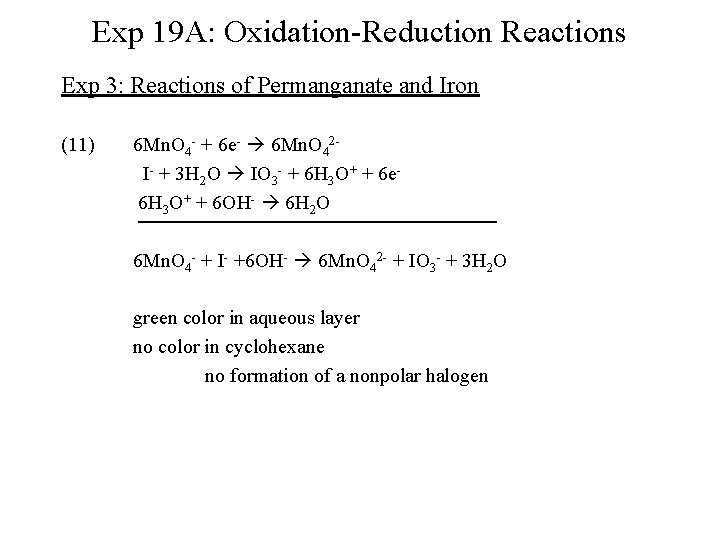
Exp 19 A: Oxidation-Reduction Reactions Exp 3: Reactions of Permanganate and Iron (11) 6 Mn. O 4 - + 6 e- 6 Mn. O 42 I- + 3 H 2 O IO 3 - + 6 H 3 O+ + 6 e 6 H 3 O+ + 6 OH- 6 H 2 O 6 Mn. O 4 - + I- +6 OH- 6 Mn. O 42 - + IO 3 - + 3 H 2 O green color in aqueous layer no color in cyclohexane no formation of a nonpolar halogen

Exp 19 A: Oxidation-Reduction Reactions Post Lab • Results sheets (p. 351 -352) • Post lab questions 1, 2 a-d, 3 a-c – Give balanced equations for every reaction that happened – If there was no reaction, write “NR” and indicate how you reached that conclusion – Answer the questions