Exp 13 The Rate of an Iodine Clock





- Slides: 13

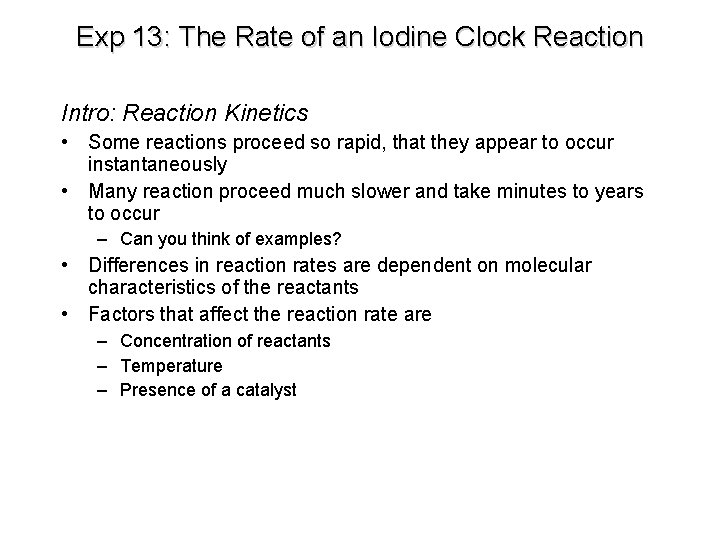
Exp 13: The Rate of an Iodine Clock Reaction Intro: Reaction Kinetics • Some reactions proceed so rapid, that they appear to occur instantaneously • Many reaction proceed much slower and take minutes to years to occur – Can you think of examples? • Differences in reaction rates are dependent on molecular characteristics of the reactants • Factors that affect the reaction rate are – Concentration of reactants – Temperature – Presence of a catalyst

Exp 13: The Rate of an Iodine Clock Reaction Purpose • Determine the rate law for the iodine clock reaction • Evaluate the effect of a catalyst on the rate law • See: Chang, Chapter 13

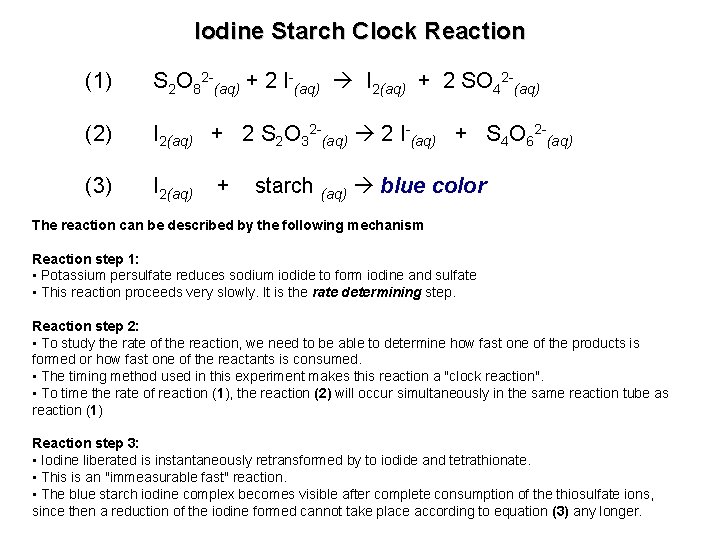
Exp 13: The Rate of an Iodine Clock Reaction • Series of redox reactions – Oxidation of iodide to iodine by potassium persulfate – Reaction of iodine with starch gives a dark-blue color • • See: Chang, Chapter 13 Rxn 1 S 2 O 82 - + 2 I- 2 SO 42 - + I 2 Rxn 2 I 2 + 2 S 2 O 32 - 2 I- + S 4 O 62 Rxn 3 I 2(aq) + starch (aq) blue color Modified Landolt Reaction

Iodine Starch Clock Reaction (1) S 2 O 82 -(aq) + 2 I-(aq) I 2(aq) + 2 SO 42 -(aq) (2) I 2(aq) + 2 S 2 O 32 -(aq) 2 I-(aq) + S 4 O 62 -(aq) (3) I 2(aq) + starch (aq) blue color The reaction can be described by the following mechanism Reaction step 1: • Potassium persulfate reduces sodium iodide to form iodine and sulfate • This reaction proceeds very slowly. It is the rate determining step. Reaction step 2: • To study the rate of the reaction, we need to be able to determine how fast one of the products is formed or how fast one of the reactants is consumed. • The timing method used in this experiment makes this reaction a "clock reaction". • To time the rate of reaction (1), the reaction (2) will occur simultaneously in the same reaction tube as reaction (1) Reaction step 3: • Iodine liberated is instantaneously retransformed by to iodide and tetrathionate. • This is an "immeasurable fast" reaction. • The blue starch iodine complex becomes visible after complete consumption of the thiosulfate ions, since then a reduction of the iodine formed cannot take place according to equation (3) any longer.

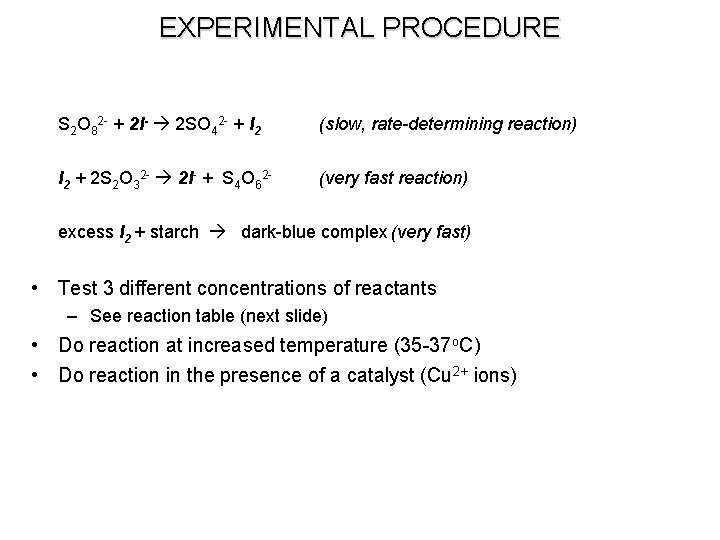
EXPERIMENTAL PROCEDURE S 2 O 82 - + 2 I- 2 SO 42 - + I 2 (slow, rate-determining reaction) I 2 + 2 S 2 O 32 - 2 I- + S 4 O 62 - (very fast reaction) excess I 2 + starch dark-blue complex (very fast) • Test 3 different concentrations of reactants – See reaction table (next slide) • Do reaction at increased temperature (35 -37 o. C) • Do reaction in the presence of a catalyst (Cu 2+ ions)

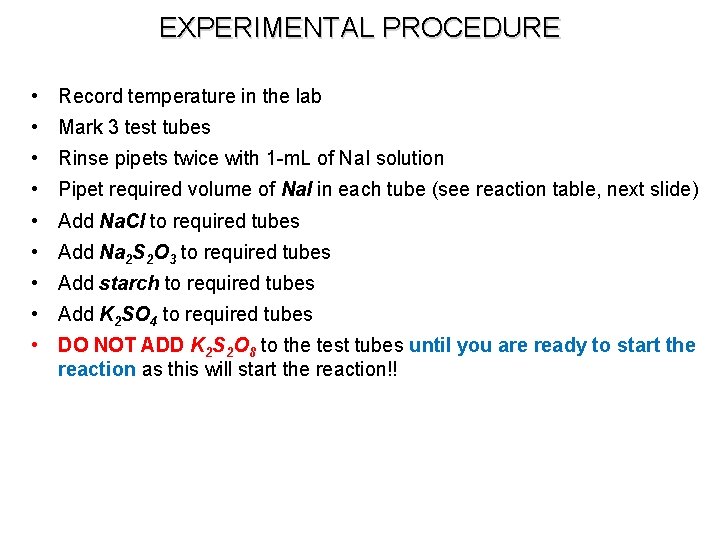
EXPERIMENTAL PROCEDURE • Record temperature in the lab • Mark 3 test tubes • Rinse pipets twice with 1 -m. L of Na. I solution • Pipet required volume of Na. I in each tube (see reaction table, next slide) • Add Na. Cl to required tubes • Add Na 2 S 2 O 3 to required tubes • Add starch to required tubes • Add K 2 SO 4 to required tubes • DO NOT ADD K 2 S 2 O 8 to the test tubes until you are ready to start the reaction as this will start the reaction!!

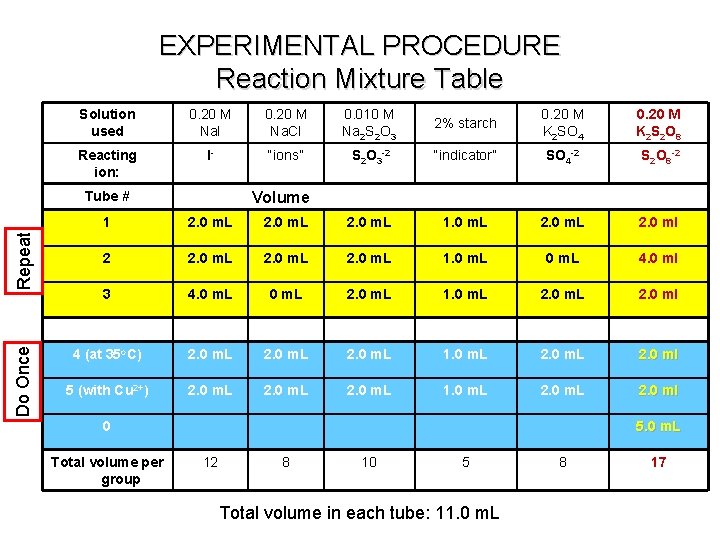
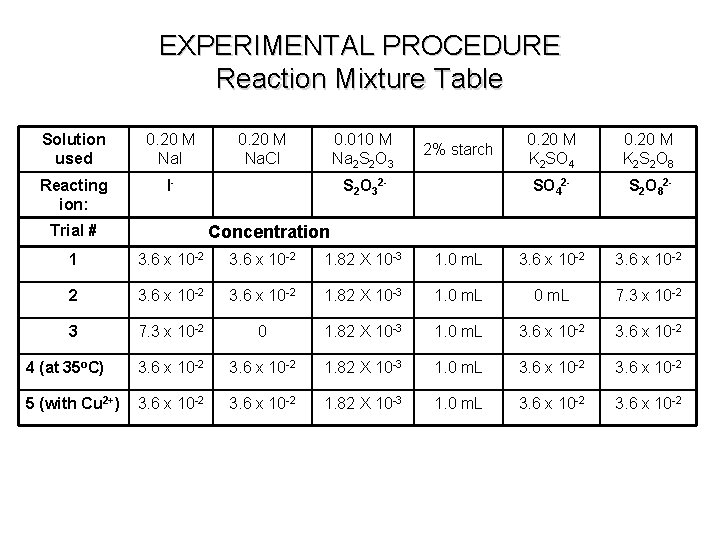
EXPERIMENTAL PROCEDURE Reaction Mixture Table Solution used 0. 20 M Na. I 0. 20 M Na. Cl 0. 010 M Na 2 S 2 O 3 2% starch 0. 20 M K 2 SO 4 0. 20 M K 2 S 2 O 8 Reacting ion: I- “ions” S 2 O 3 -2 “indicator” SO 4 -2 S 2 O 8 -2 Do Once Repeat Tube # Volume 1 2. 0 m. L 1. 0 m. L 2. 0 ml 2 2. 0 m. L 1. 0 m. L 4. 0 ml 3 4. 0 m. L 2. 0 m. L 1. 0 m. L 2. 0 ml 4 (at 35 o. C) 2. 0 m. L 1. 0 m. L 2. 0 ml 5 (with Cu 2+) 2. 0 m. L 1. 0 m. L 2. 0 ml 0 Total volume per group 5. 0 m. L 12 8 10 5 Total volume in each tube: 11. 0 m. L 8 17

EXPERIMENTAL PROCEDURE • Experiment #1 – Note time or use stopwatch – Add 2. 0 m. L K 2 S 2 O 8 to tube #1 • this will start the reaction!! – Quickly cover tube with Parafilm and invert ~ 3 -5 times to mix – Record the time that the dark color appears • The color appears suddenly!! – Exp #1 should take about 4 -8 minutes • Experiment #2 – Repeat the experiment with tube #2 – Add 4. 0 m. L K 2 S 2 O 8 to tube #2 – Mix as before and record how long it takes for the solution to turn blue • Experiment #3 – Repeat the experiment with tube #3 – Add 2. 0 m. L K 2 S 2 O 8 to tube #3 – Mix as before and record how long it takes for the solution to turn blue • • Clean and dry the tubes and repeat the experiments Differences in time should be less than 10 s

EXPERIMENTAL PROCEDURE • Experiment #4: temperature effect – Put warm water from the faucet in a beaker – Pipet ~ 5 m. L of K 2 S 2 O 8 in tube #0 – Pipet the volumes in tube #4 as indicated in the reaction table, except K 2 S 2 O 8 – Put the tubes in the warm water bath – Allow to equilibrate for ~ 5 min – Start the reaction by adding 2 m. L K 2 S 2 O 8 from tube #0 into tube #4 – Note time and mix as before – Put tube back into water bath – Record time to turn blue • Experiment #5 – Fill tube #5 with the same amounts as tube #1, except K 2 S 2 O 8 (see reaction table) – Add 1 drop of 0. 2 M Cu. SO 4 solution – Shake gently to mix – Initiate reaction by adding 2. 0 m. L K 2 S 2 O 8 (room temp, not warm!!) – Mix as before and record how long it takes for the solution to turn blue

EXPERIMENTAL PROCEDURE Reaction Mixture Table Solution used 0. 20 M Na. I Reacting ion: I- Trial # 0. 20 M Na. Cl 0. 010 M Na 2 S 2 O 3 2% starch S 2 O 32 - 0. 20 M K 2 SO 4 0. 20 M K 2 S 2 O 8 SO 42 - S 2 O 82 - Concentration 1 3. 6 x 10 -2 1. 82 X 10 -3 1. 0 m. L 3. 6 x 10 -2 2 3. 6 x 10 -2 1. 82 X 10 -3 1. 0 m. L 7. 3 x 10 -2 3 7. 3 x 10 -2 0 1. 82 X 10 -3 1. 0 m. L 3. 6 x 10 -2 4 (at 35 o. C) 3. 6 x 10 -2 1. 82 X 10 -3 1. 0 m. L 3. 6 x 10 -2 5 (with Cu 2+) 3. 6 x 10 -2 1. 82 X 10 -3 1. 0 m. L 3. 6 x 10 -2

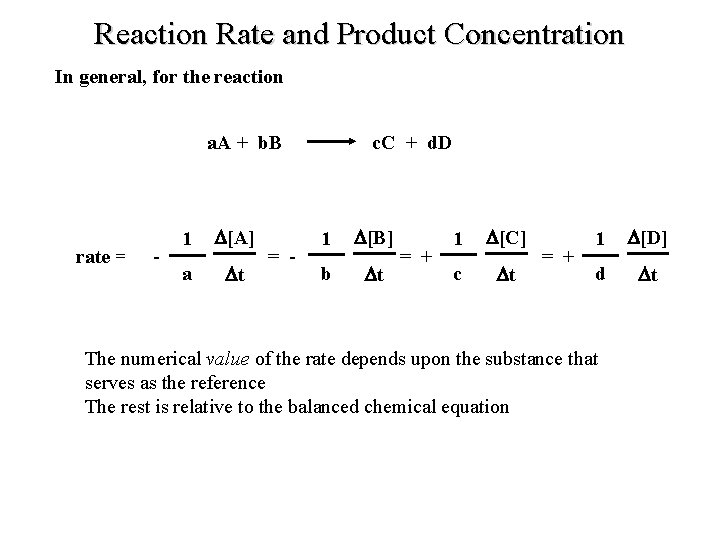
Reaction Rate and Product Concentration In general, for the reaction a. A + b. B rate = - 1 [A] a t = - c. C + d. D 1 [B] b t = + 1 [C] c t = + 1 [D] d t The numerical value of the rate depends upon the substance that serves as the reference The rest is relative to the balanced chemical equation

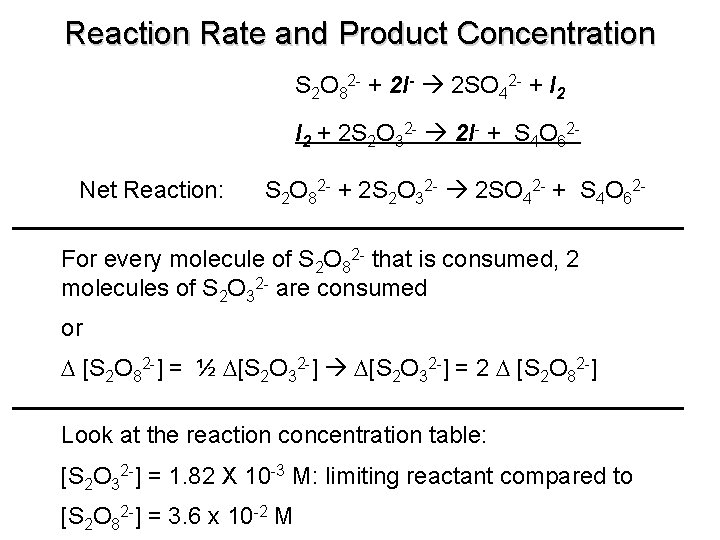
Reaction Rate and Product Concentration S 2 O 82 - + 2 I- 2 SO 42 - + I 2 + 2 S 2 O 32 - 2 I- + S 4 O 62 Net Reaction: S 2 O 82 - + 2 S 2 O 32 - 2 SO 42 - + S 4 O 62 - For every molecule of S 2 O 82 - that is consumed, 2 molecules of S 2 O 32 - are consumed or D [S 2 O 82 -] = ½ D[S 2 O 32 -] = 2 D [S 2 O 82 -] Look at the reaction concentration table: [S 2 O 32 -] = 1. 82 X 10 -3 M: limiting reactant compared to [S 2 O 82 -] = 3. 6 x 10 -2 M

Lab Report for Exp 13” “The Rate of an Iodine Clock Reaction” Due Monday Oct 15: – Data Sheets, calculations – Post-lab question 1 a-e, 3 – Post-lab question 2 (5 bonus points) Next Week: Exp 14 A – Le Chatelier’s Principle Prelab Preparations for Exp 14 A: “Le Chatelier’s Principle” – Due: Prelab questions: #1, 2, 3, 4, 5 – For more info, see Chang, Chapter 14 – Lab preparations as usual: Read and look up the following • Introduction and purpose of the experiment • Experimental procedure • Properties of chemicals – Ammonia, NH 3. Nickel nitrate, Ni(NO 3)2 – Sodium hydroxide, Na. OH. Cobalt nitrate, Co(NO 3)2 – Hydrochloric acid, HCl. Calcium nitrate, Ca(NO 3)2 .