Exothermic and Endothermic Reactions Oxidation Reactions Exothermic vs









- Slides: 14

Exothermic and Endothermic Reactions Oxidation Reactions

Exothermic vs Endothermic

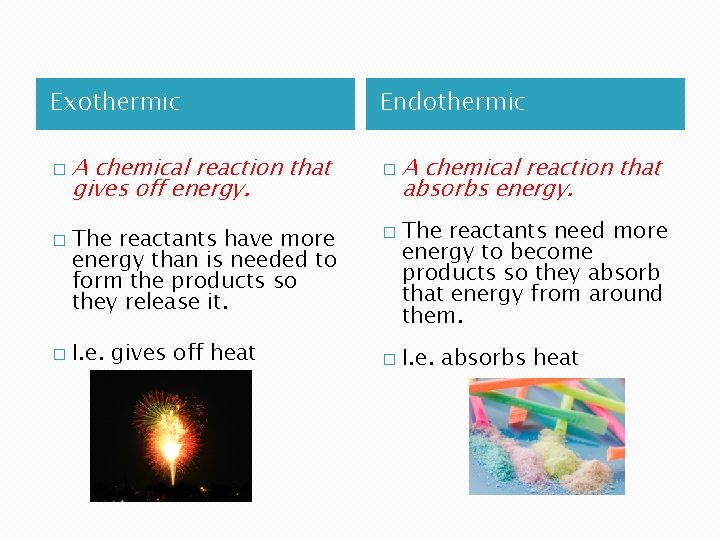
Exothermic � � � A chemical reaction that gives off energy. The reactants have more energy than is needed to form the products so they release it. I. e. gives off heat Endothermic � � � A chemical reaction that absorbs energy. The reactants need more energy to become products so they absorb that energy from around them. I. e. absorbs heat

Endothermic Reaction � Collect: ◦ ◦ ◦ ½ teaspoon of citric acid (C 6 H 8 O 7) ¼ teaspoon baking soda (Na. HCO 3) 3 teaspoons of icing sugar (C 12 H 22 O 11) Clean mixing container Teaspoon � Do this: ◦ Add all ingredients to the small mixing container. ◦ Use the back of the teaspoon to crush any lumps as you mix together. ◦ Keep it dry until ready to eat!

Oxidation Reactions

Combustion

Combustion � Combustion is an exothermic reaction. � Combustion occurs whenever something reacts with oxygen gas (O 2) � No oxygen = no combustion

Burning Steel Wool Example 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) You. Tube Video: www. youtube. com/watch? v=B 6 MX 5 uy. Xrb 4

Complete vs Incomplete Combustion

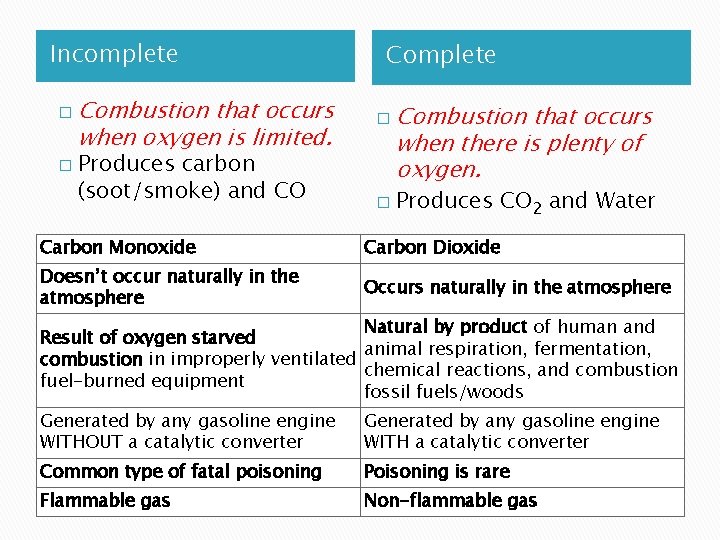
Incomplete � � Combustion that occurs when oxygen is limited. Produces carbon (soot/smoke) and CO Complete � � Combustion that occurs when there is plenty of oxygen. Produces CO 2 and Water Carbon Monoxide Carbon Dioxide Doesn’t occur naturally in the atmosphere Occurs naturally in the atmosphere Natural by product of human and Result of oxygen starved animal respiration, fermentation, combustion in improperly ventilated chemical reactions, and combustion fuel-burned equipment fossil fuels/woods Generated by any gasoline engine WITHOUT a catalytic converter Generated by any gasoline engine WITH a catalytic converter Common type of fatal poisoning Poisoning is rare Flammable gas Non-flammable gas

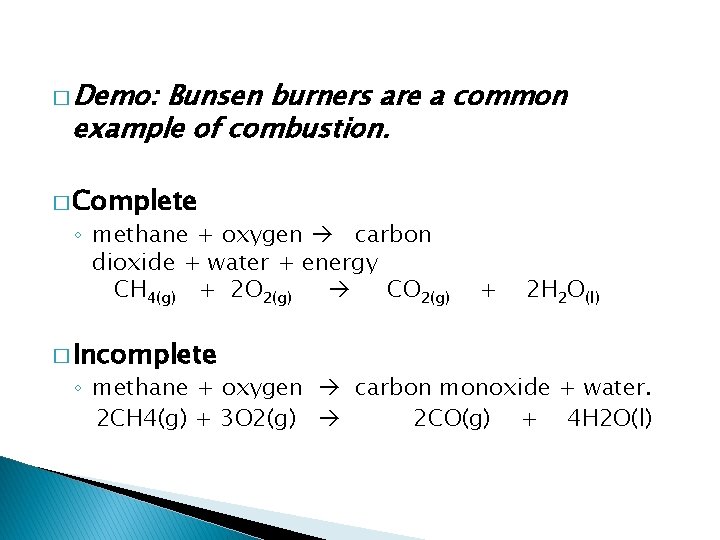
� Demo: Bunsen burners are a common example of combustion. � Complete ◦ methane + oxygen carbon dioxide + water + energy CH 4(g) + 2 O 2(g) CO 2(g) � Incomplete + 2 H 2 O(l) ◦ methane + oxygen carbon monoxide + water. 2 CH 4(g) + 3 O 2(g) 2 CO(g) + 4 H 2 O(l)

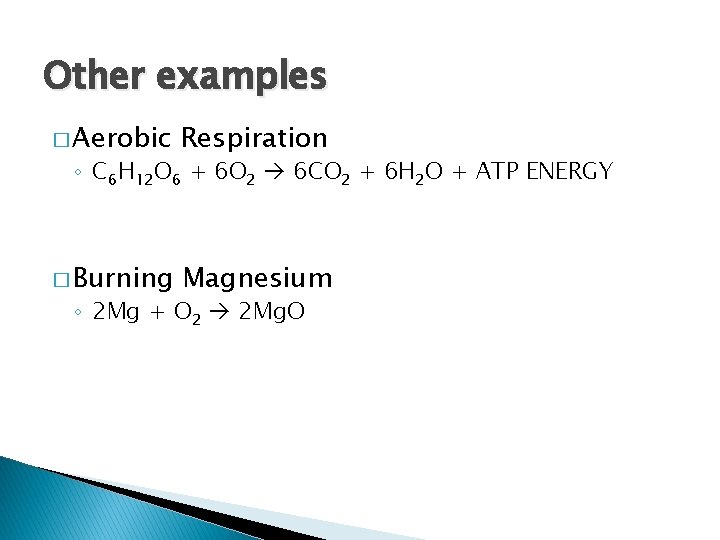
Other examples � Aerobic Respiration � Burning Magnesium ◦ C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ATP ENERGY ◦ 2 Mg + O 2 2 Mg. O

Corrosion

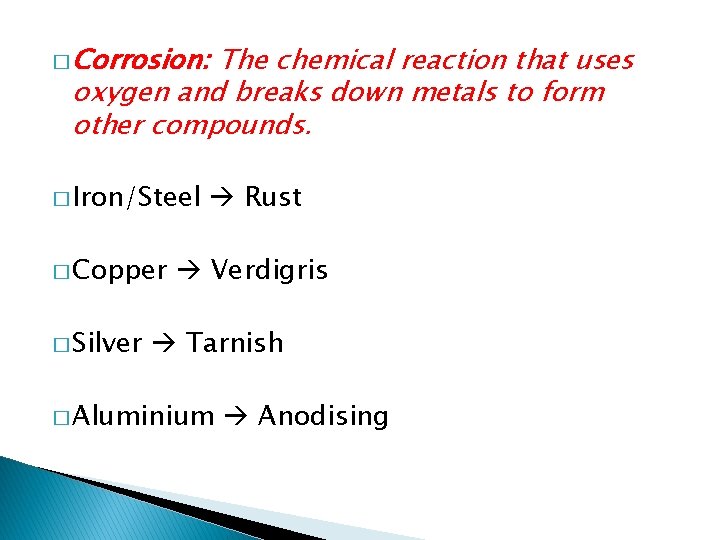
� Corrosion: The chemical reaction that uses oxygen and breaks down metals to form other compounds. � Iron/Steel � Copper � Silver Rust Verdigris Tarnish � Aluminium Anodising