Exothermic and Endothermic reactions Learning Objectives Explain heat

Exothermic and Endothermic reactions

Learning Objectives �Explain heat as a transfer of energy �Differentiate between heat and temperature �Describe exothermic and endothermic reactions �Investigate dissolving of salts as exothermic and endothermic processes
Heat �Heat is a thermal energy transferred from hotter system to cooler system that are in contact with each other. Is there any difference between heat and temperature?
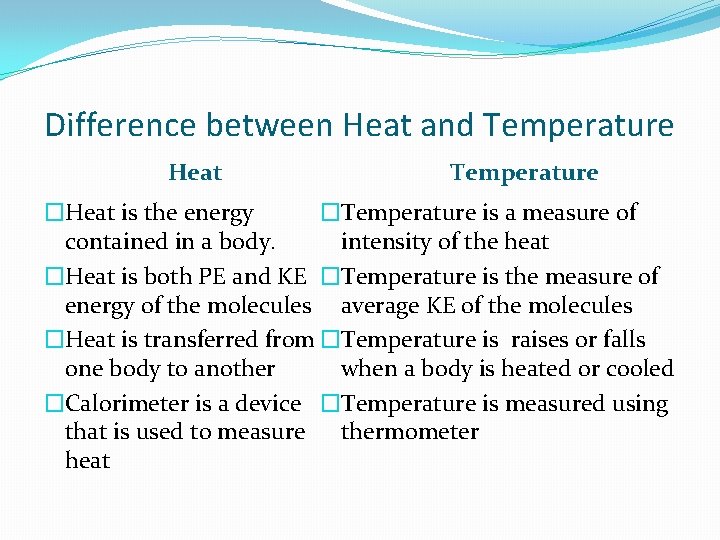
Difference between Heat and Temperature Heat Temperature �Heat is the energy �Temperature is a measure of contained in a body. intensity of the heat �Heat is both PE and KE �Temperature is the measure of energy of the molecules average KE of the molecules �Heat is transferred from �Temperature is raises or falls one body to another when a body is heated or cooled �Calorimeter is a device �Temperature is measured using that is used to measure thermometer heat
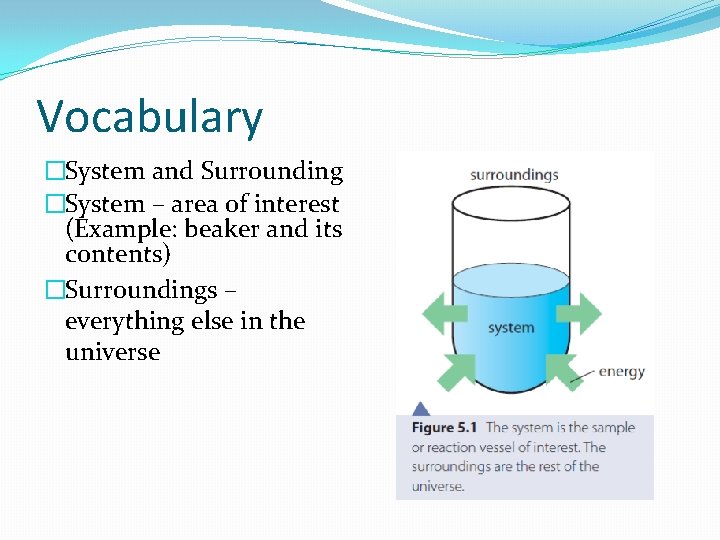
Vocabulary �System and Surrounding �System – area of interest (Example: beaker and its contents) �Surroundings – everything else in the universe

�An “open” system can exchange matter and energy with the surroundings. �A “closed” system can only exchange energy, not matter, with the surroundings. �An “Isolated” system – Neither heat not matter is exchanged with surrounding
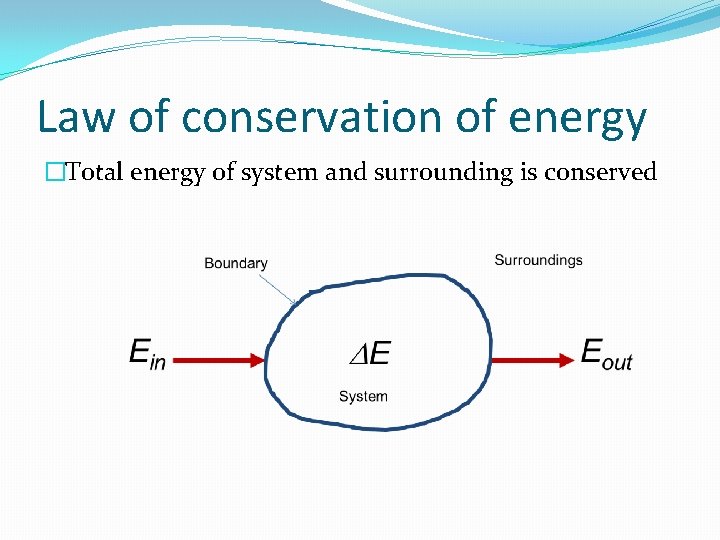
Law of conservation of energy �Total energy of system and surrounding is conserved
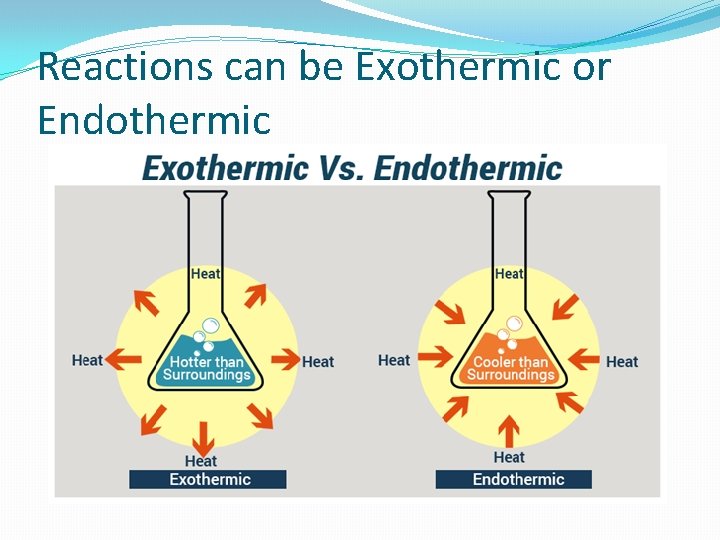
Reactions can be Exothermic or Endothermic

Exothermic Reactions Think of some examples of exothermic reactions in daily life

Here are some examples �Firework �Condensation of rain from water vapor �Cement and concrete setting �Combustion of fuels

Endothermic reactions Think of some examples of Endothermic reactions in daily life
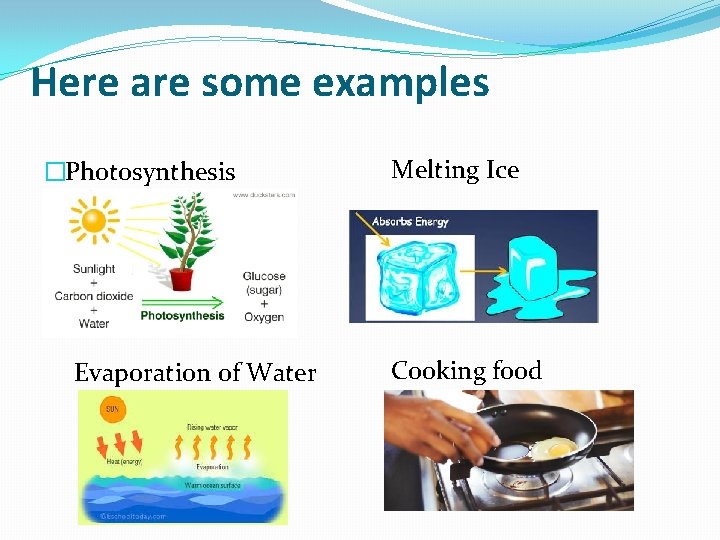
Here are some examples �Photosynthesis Evaporation of Water Melting Ice Cooking food
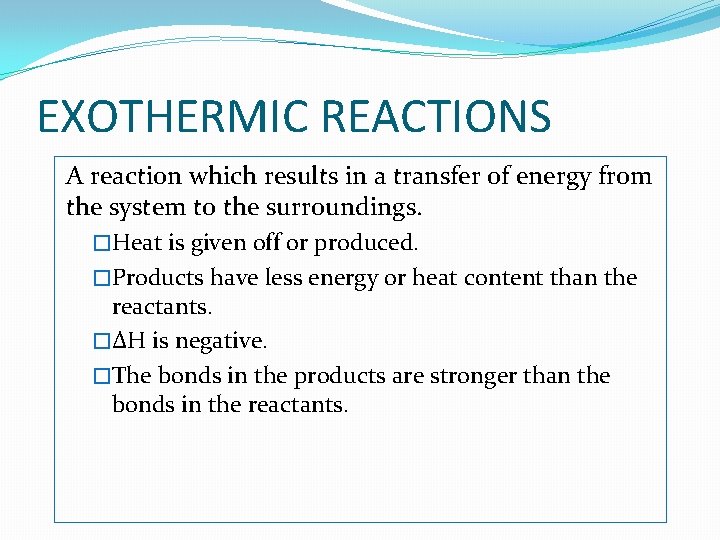
EXOTHERMIC REACTIONS A reaction which results in a transfer of energy from the system to the surroundings. �Heat is given off or produced. �Products have less energy or heat content than the reactants. �ΔH is negative. �The bonds in the products are stronger than the bonds in the reactants.
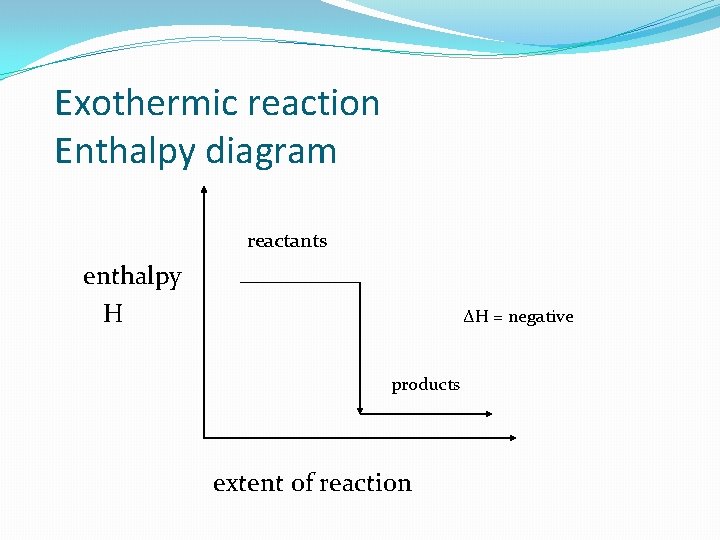
Exothermic reaction Enthalpy diagram reactants enthalpy H ΔH = negative products extent of reaction
ENDOTHERMIC REACTIONS �A reaction which results in a transfer of energy from the surroundings to the system. �Heat is absorbed. �Reactants have less energy than the products. �ΔH is positive. �The bonds in the reactants are stronger than those in the products.
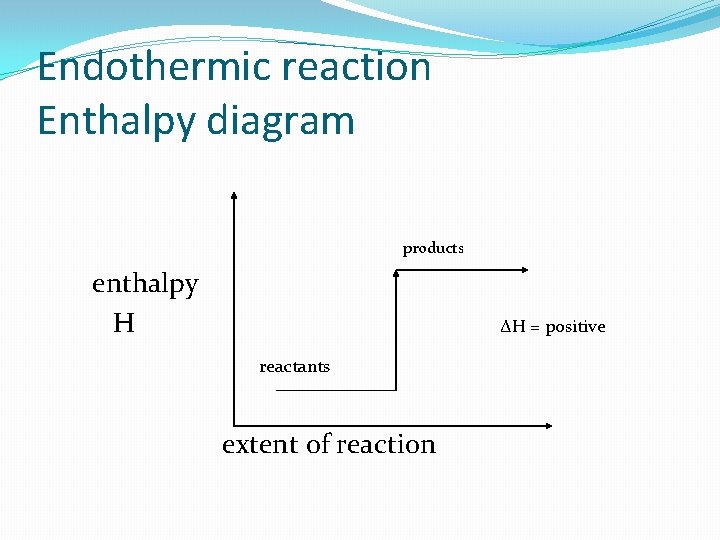
Endothermic reaction Enthalpy diagram products enthalpy H ΔH = positive reactants extent of reaction

Investigation
- Slides: 17