Exothermic and endothermic reactions Chemical Reactions usually involve






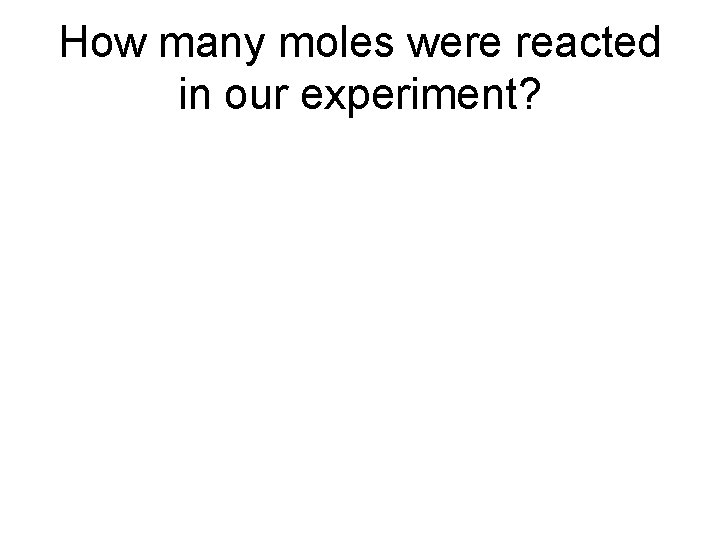























- Slides: 101

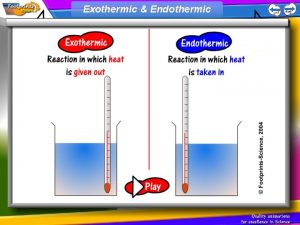
Exothermic and endothermic reactions

• Chemical Reactions usually involve a temperature change (heat is given out or taken in)

Law of conservation of energy • Energy cannot be created or destroyed, but only changed from one form into another

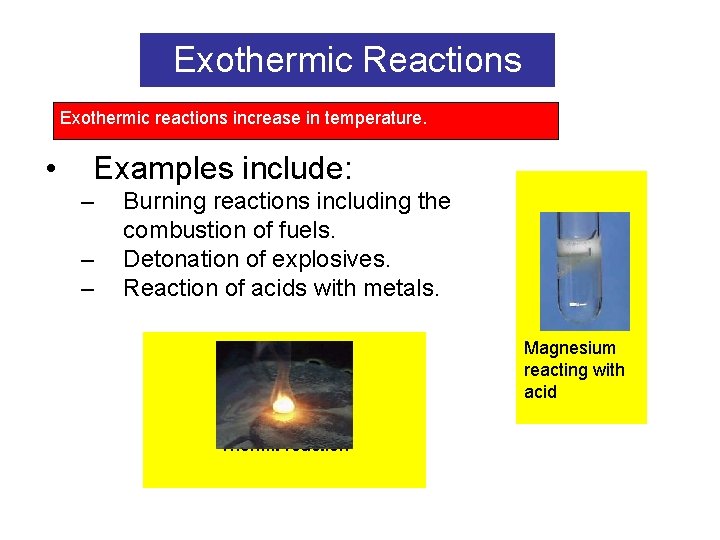
Exothermic Reactions Exothermic reactions increase in temperature. • Examples include: – – – Burning reactions including the combustion of fuels. Detonation of explosives. Reaction of acids with metals. Magnesium reacting with acid Thermit reaction

Exothermic Reactions • Magnesium + Hydrochloric acid 25 o C 45 o C magnesium Gets hot Hydrochloric acid Heat energy given out

Exothermic Reactions 45 25 oo C Reactants convert chemical energy to heat energy. The temperature rises.

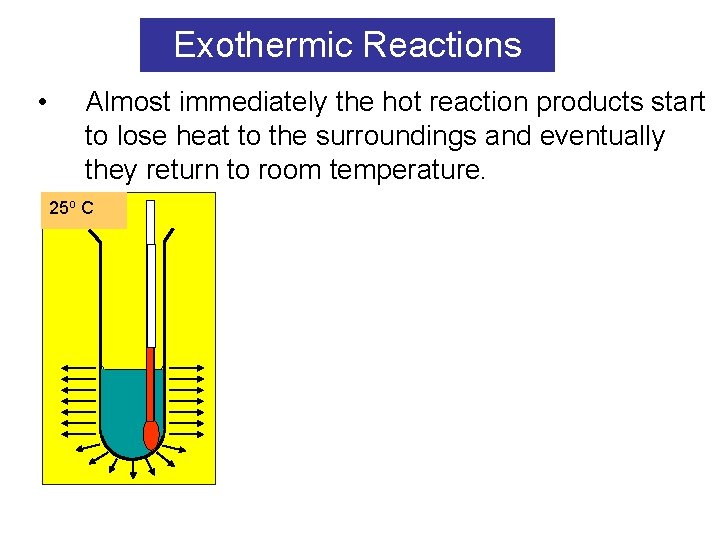
Exothermic Reactions • Almost immediately the hot reaction products start to lose heat to the surroundings and eventually they return to room temperature. 25 o C 45

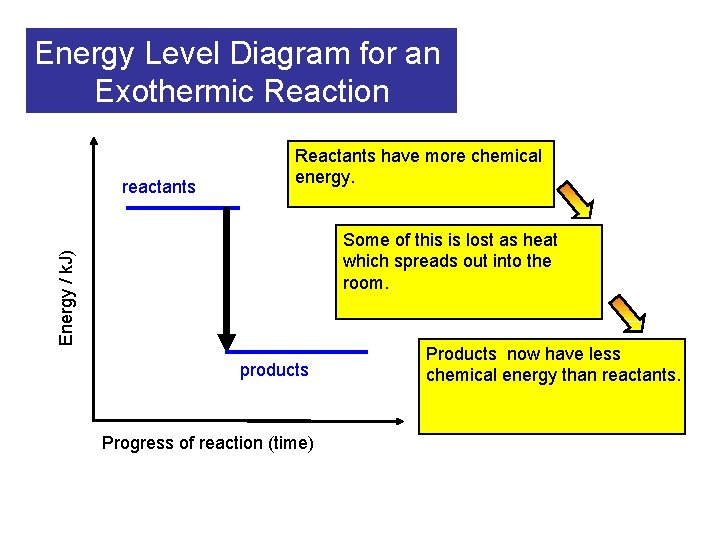
Energy Level Diagram for an Exothermic Reaction reactants Reactants have more chemical energy. Energy / k. J) Some of this is lost as heat which spreads out into the room. products Progress of reaction (time) Products now have less chemical energy than reactants.

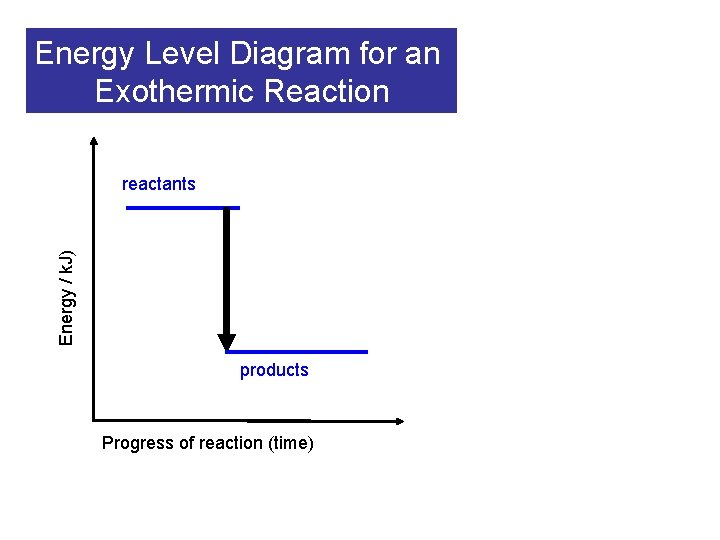
Energy Level Diagram for an Exothermic Reaction Energy / k. J) reactants products Progress of reaction (time)

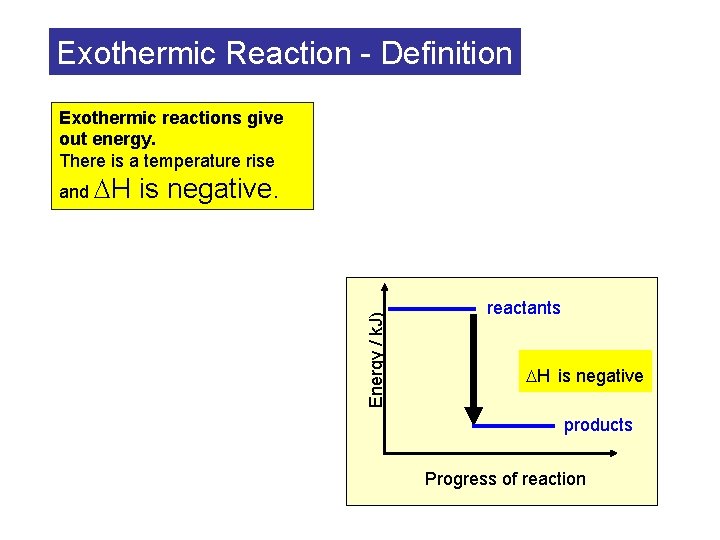
Exothermic Reaction - Definition Exothermic reactions give out energy. There is a temperature rise is negative. Energy / k. J) and H reactants H is negative products Progress of reaction

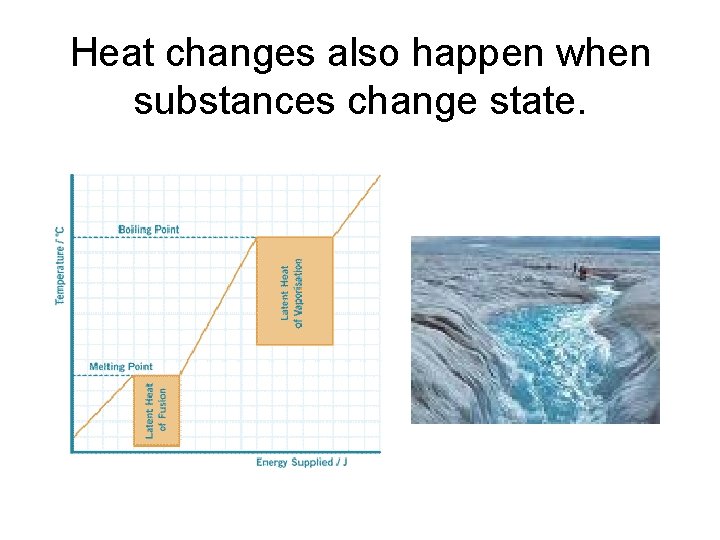
Heat changes also happen when substances change state.

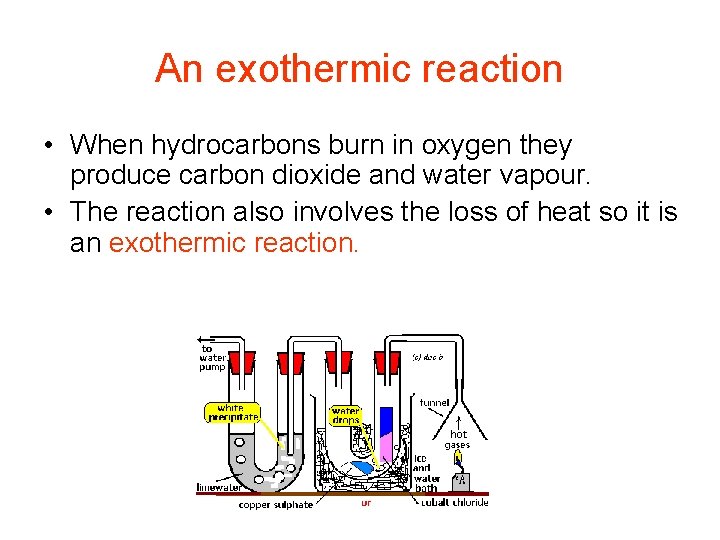
An exothermic reaction • When hydrocarbons burn in oxygen they produce carbon dioxide and water vapour. • The reaction also involves the loss of heat so it is an exothermic reaction.

Objectives for today. . • Define endothermic reaction • Draw an energy profile diagram for an endothermic reaction • Bond energy definition • Mandatory experiment Homework – Write up today’s experiment in experiment copy

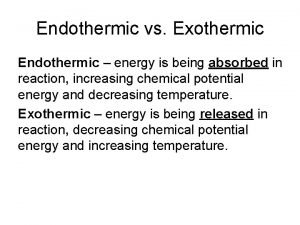
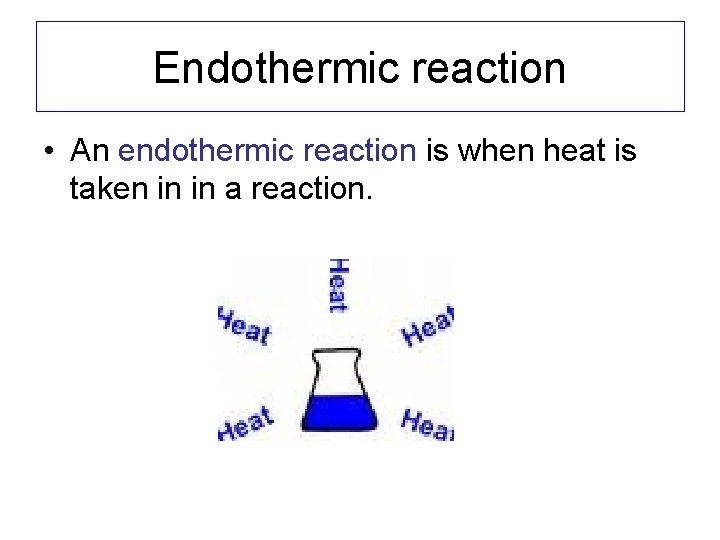
Endothermic reaction • An endothermic reaction is when heat is taken in in a reaction.

Endothermic Reactions • • Endothermic chemical reactions are relatively rare. A few reactions that give off gases are highly endothermic - get very cold.

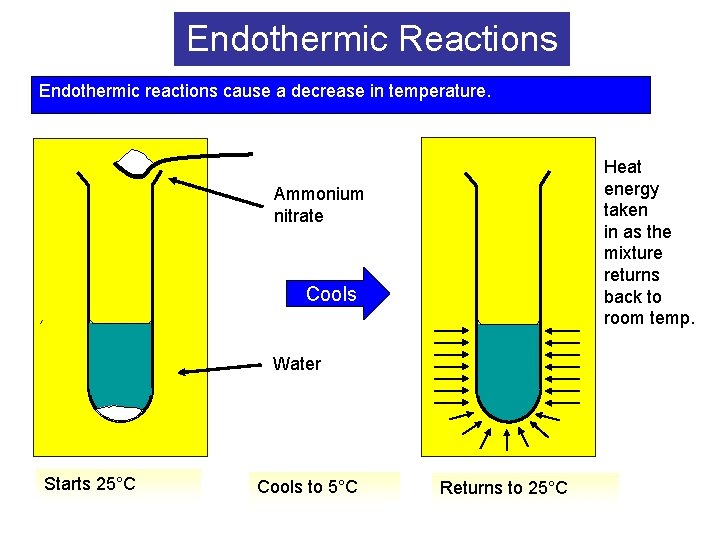
Endothermic Reactions Endothermic reactions cause a decrease in temperature. Heat energy taken in as the mixture returns back to room temp. Ammonium nitrate Cools Water Starts 25°C Cools to 5°C Returns to 25°C

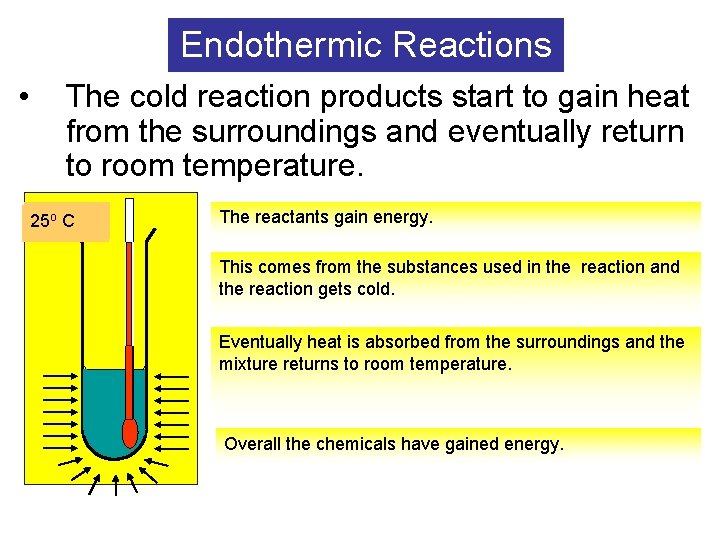
Endothermic Reactions • The cold reaction products start to gain heat from the surroundings and eventually return to room temperature. o oo. CC 25 5 The reactants gain energy. This comes from the substances used in the reaction and the reaction gets cold. Eventually heat is absorbed from the surroundings and the mixture returns to room temperature. Overall the chemicals have gained energy.

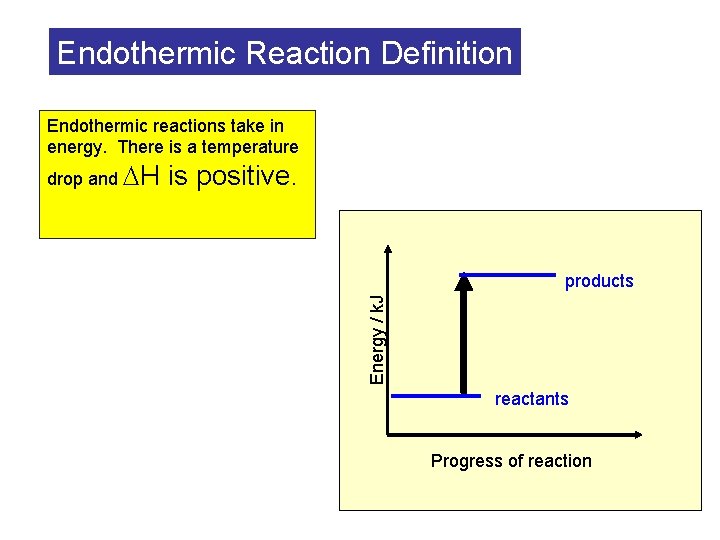
Endothermic Reaction Definition Endothermic reactions take in energy. There is a temperature is positive. products Energy / k. J drop and H reactants Progress of reaction

Homework • Revision CH 1, 2, 3 HL 2015 Q 4 a, b Q 5 a, c 2014 Q 4 b, Q 10 b OL 2015 Q 4 a 2013 Q 5 b 2011 Q 4 a, 2010 Q 4 a 2009 Q 4 b 2008 Q 11 a

Heat of reaction • The heat of reaction is the heat change when the number of moles of reactants indicated in the balanced equation react completely. For an exothermic reaction: the heat of reaction is always negative e. g ∆H = -34 k. J For an endothermic reaction: the heat of reaction is always positive e. g ∆H = +34 k. J


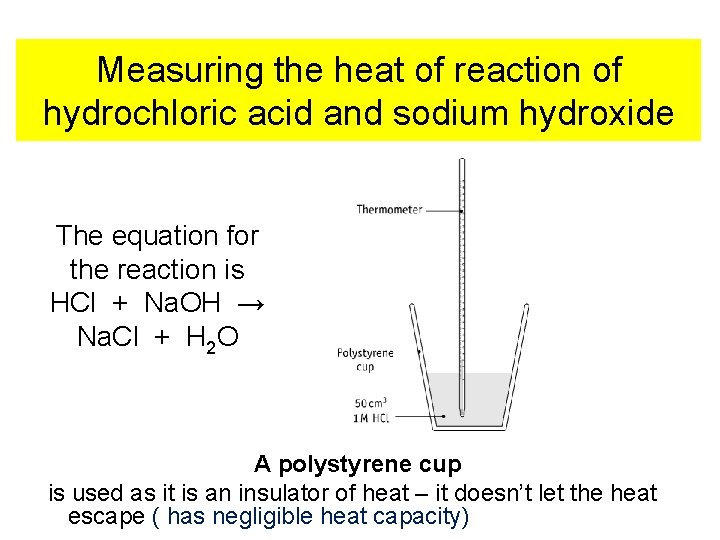
Measuring the heat of reaction of hydrochloric acid and sodium hydroxide The equation for the reaction is HCl + Na. OH → Na. Cl + H 2 O A polystyrene cup is used as it is an insulator of heat – it doesn’t let the heat escape ( has negligible heat capacity)

Other precautions to ensure an accurate result! 1. Make sure both solutions are at the same temperature before you start! 2. Wash thermometer and dry it before switching solutions. 3. Stir the mixture slowly and make sure none of the mixture is splashed out of the cup.

Results

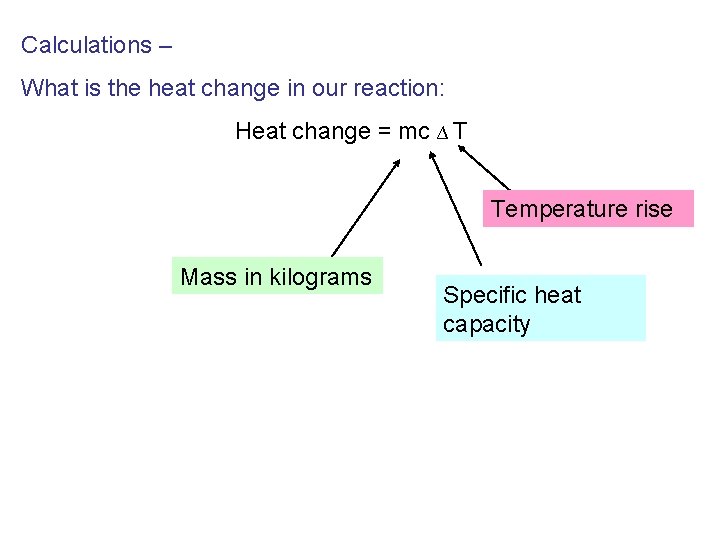
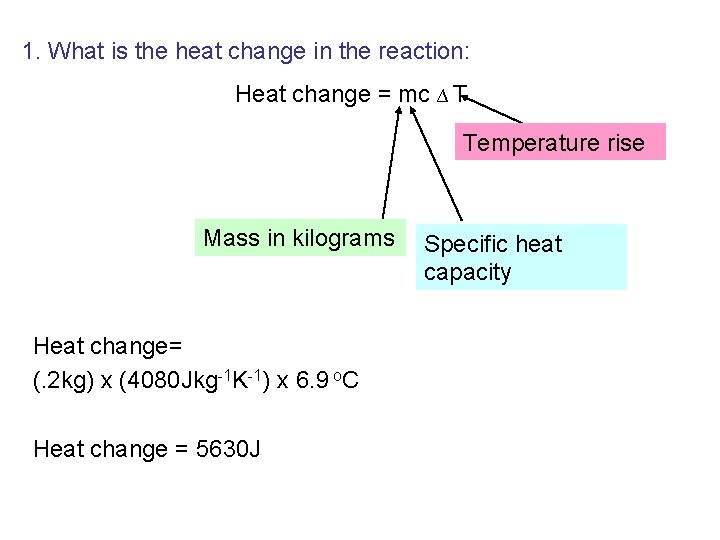
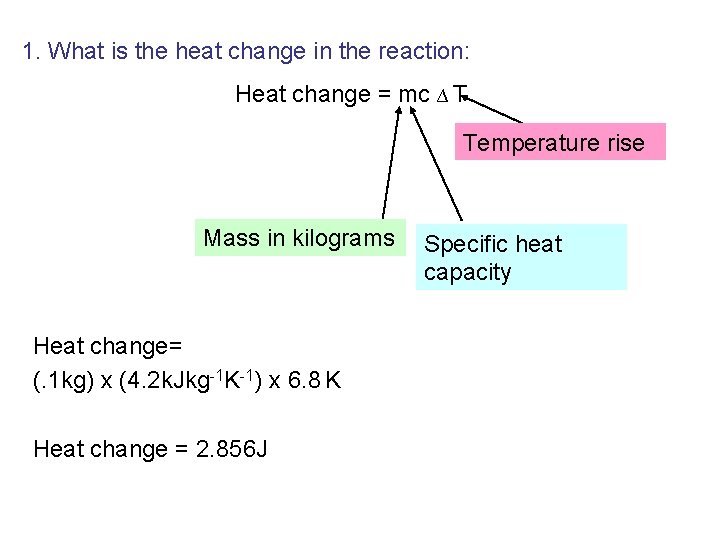
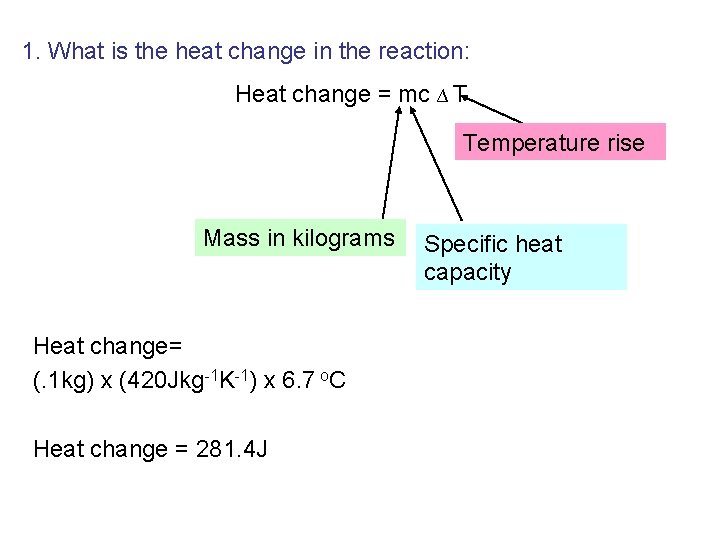
Calculations – What is the heat change in our reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Specific heat capacity
How many moles were reacted in our experiment?

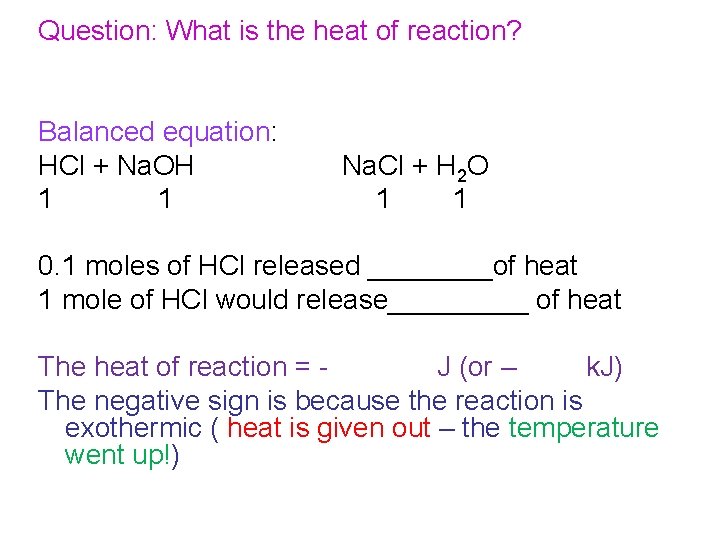
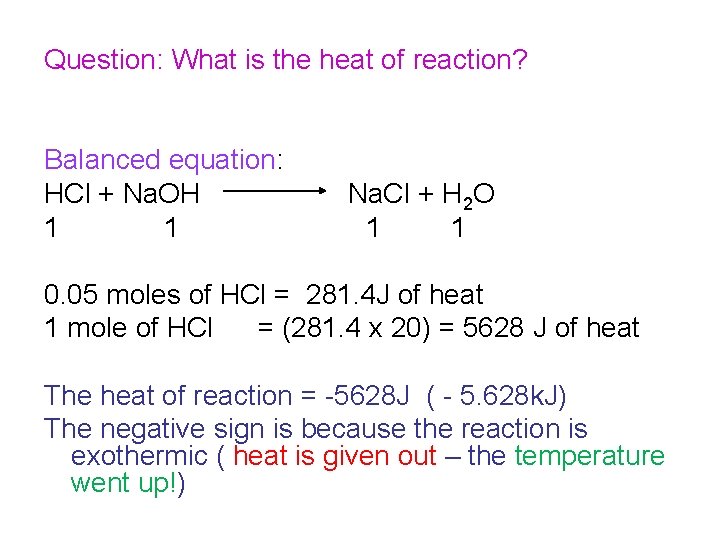
Question: What is the heat of reaction? Balanced equation: HCl + Na. OH 1 1 Na. Cl + H 2 O 1 1 0. 1 moles of HCl released ____of heat 1 mole of HCl would release_____ of heat The heat of reaction = J (or – k. J) The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

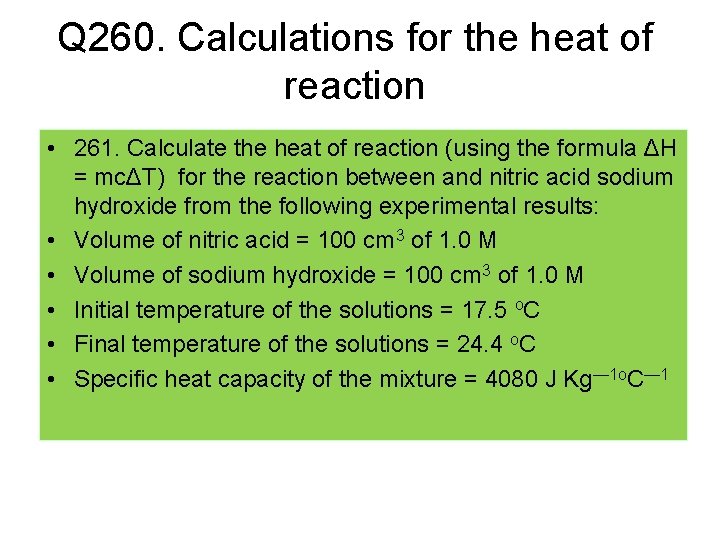
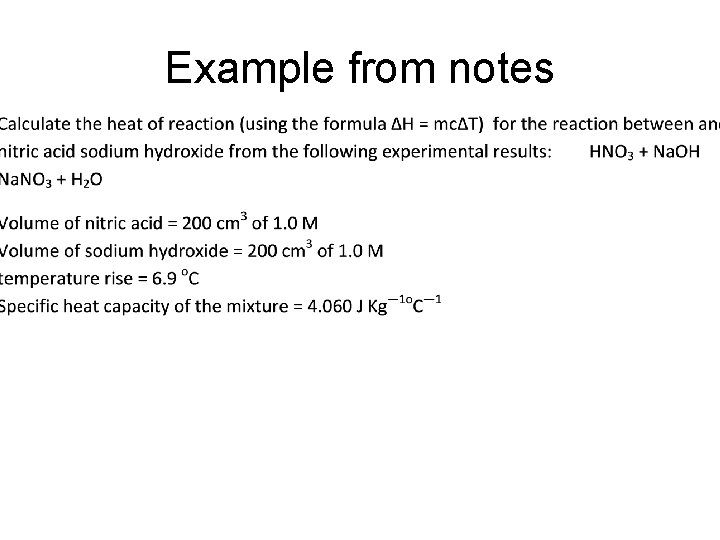
Q 260. Calculations for the heat of reaction • 261. Calculate the heat of reaction (using the formula ΔH = mcΔT) for the reaction between and nitric acid sodium hydroxide from the following experimental results: • Volume of nitric acid = 100 cm 3 of 1. 0 M • Volume of sodium hydroxide = 100 cm 3 of 1. 0 M • Initial temperature of the solutions = 17. 5 o. C • Final temperature of the solutions = 24. 4 o. C • Specific heat capacity of the mixture = 4080 J Kg— 1 o. C— 1

1. What is the heat change in the reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Heat change= (. 2 kg) x (4080 Jkg-1 K-1) x 6. 9 o. C Heat change = 5630 J Specific heat capacity

2. How many moles of Nitric acid were reacted? : • 100 cm 3 of a 1 M solution of HNO 3 was reacted 1 x 1000 = 0. 1 moles Number of moles of HNO 3 reacted = 0. 1 moles

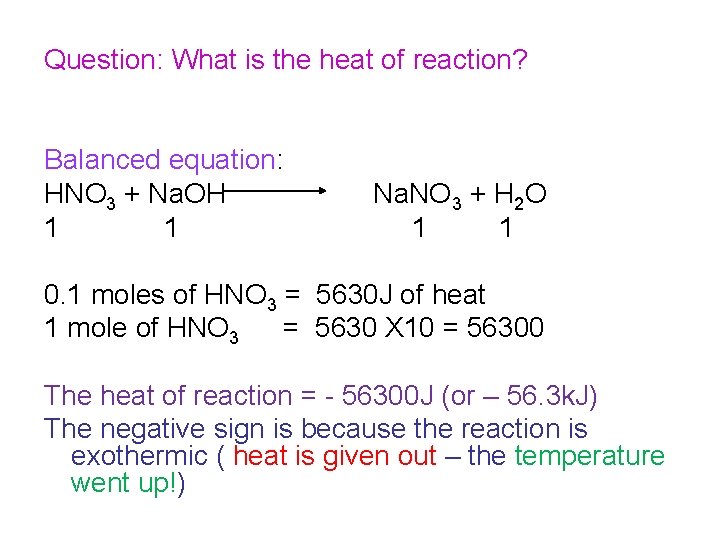
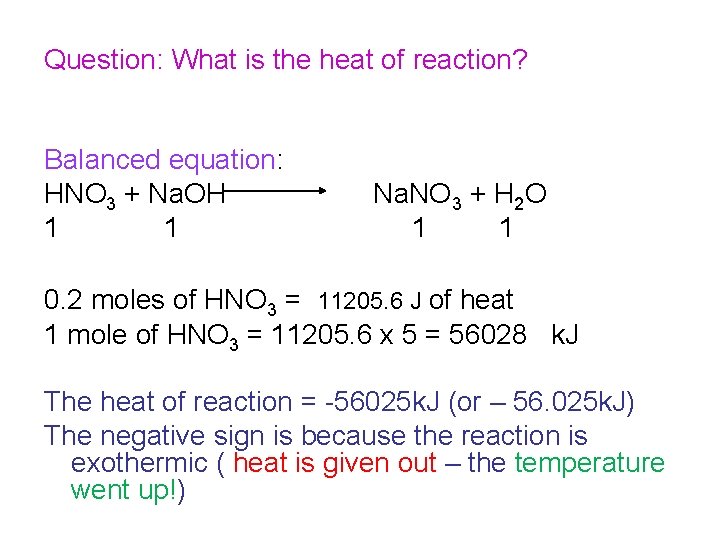
Question: What is the heat of reaction? Balanced equation: HNO 3 + Na. OH 1 1 Na. NO 3 + H 2 O 1 1 0. 1 moles of HNO 3 = 5630 J of heat 1 mole of HNO 3 = 5630 X 10 = 56300 The heat of reaction = - 56300 J (or – 56. 3 k. J) The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

Example from notes

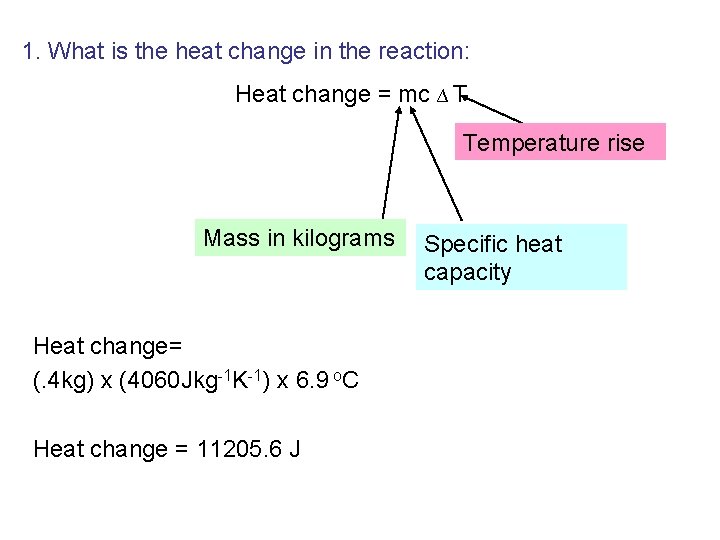
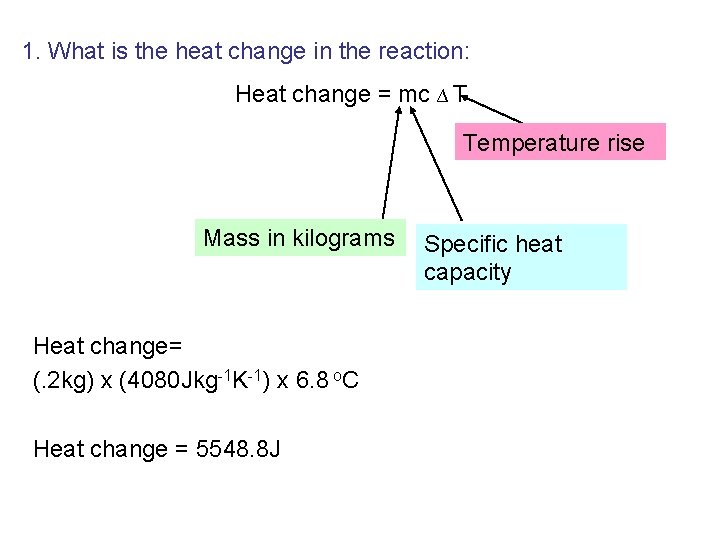
1. What is the heat change in the reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Heat change= (. 4 kg) x (4060 Jkg-1 K-1) x 6. 9 o. C Heat change = 11205. 6 J Specific heat capacity

2. How many moles of Nitric acid were reacted? : 200 cm 3 of a 1 M solution of HNO 3 was reacted (1) = 0. 2 MOLES 1000 Number of moles of HNO 3 reacted = 0. 2 moles

Question: What is the heat of reaction? Balanced equation: HNO 3 + Na. OH 1 1 Na. NO 3 + H 2 O 1 1 0. 2 moles of HNO 3 = 11205. 6 J of heat 1 mole of HNO 3 = 11205. 6 x 5 = 56028 k. J The heat of reaction = -56025 k. J (or – 56. 025 k. J) The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

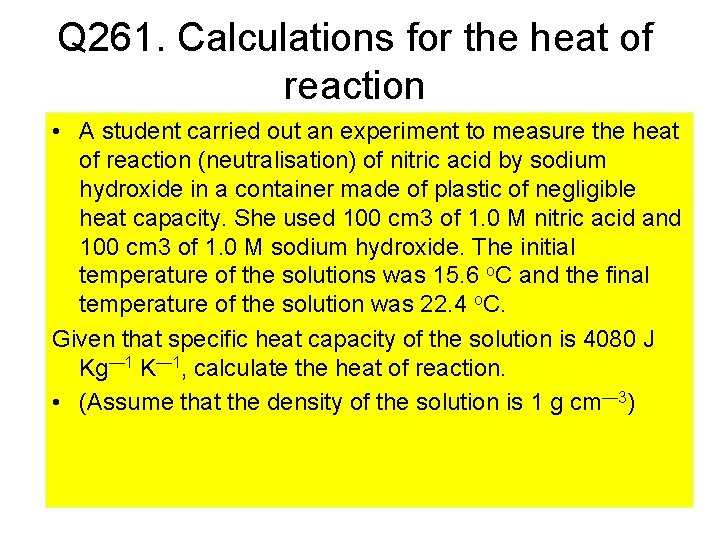
Q 261. Calculations for the heat of reaction • A student carried out an experiment to measure the heat of reaction (neutralisation) of nitric acid by sodium hydroxide in a container made of plastic of negligible heat capacity. She used 100 cm 3 of 1. 0 M nitric acid and 100 cm 3 of 1. 0 M sodium hydroxide. The initial temperature of the solutions was 15. 6 o. C and the final temperature of the solution was 22. 4 o. C. Given that specific heat capacity of the solution is 4080 J Kg— 1 K— 1, calculate the heat of reaction. • (Assume that the density of the solution is 1 g cm— 3)

1. What is the heat change in the reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Heat change= (. 2 kg) x (4080 Jkg-1 K-1) x 6. 8 o. C Heat change = 5548. 8 J Specific heat capacity

2. How many moles of Nitric acid were reacted? : • 100 cm 3 of a 1 M solution of HNO 3 was reacted • 1000 cm 3 of solution = 1 mole in it. • 100 cm 3 of solution = x moles (1) = x 10 Number of moles of HNO 3 reacted = 0. 1 moles

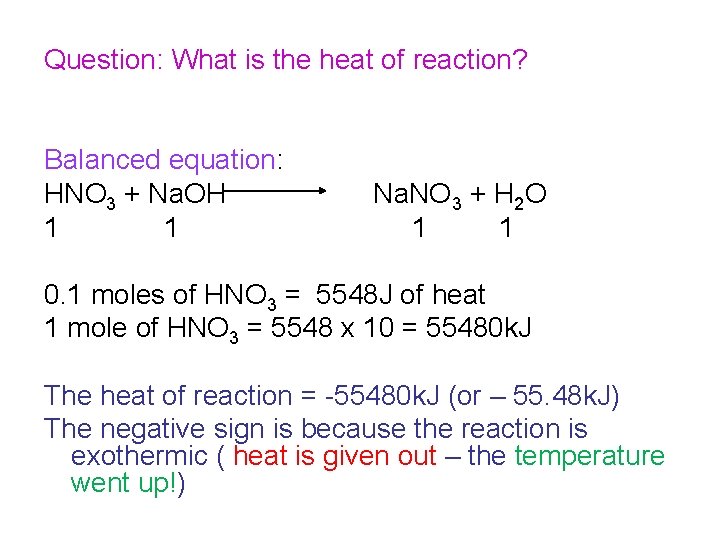
Question: What is the heat of reaction? Balanced equation: HNO 3 + Na. OH 1 1 Na. NO 3 + H 2 O 1 1 0. 1 moles of HNO 3 = 5548 J of heat 1 mole of HNO 3 = 5548 x 10 = 55480 k. J The heat of reaction = -55480 k. J (or – 55. 48 k. J) The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

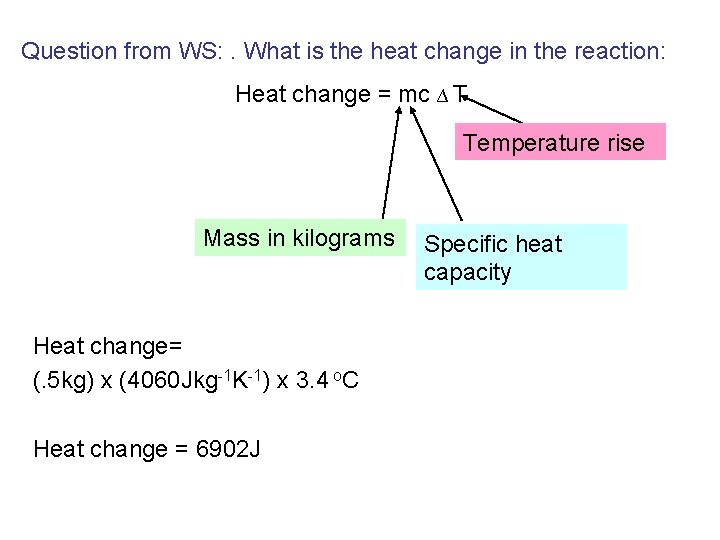
Question from WS: . What is the heat change in the reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Heat change= (. 5 kg) x (4060 Jkg-1 K-1) x 3. 4 o. C Heat change = 6902 J Specific heat capacity

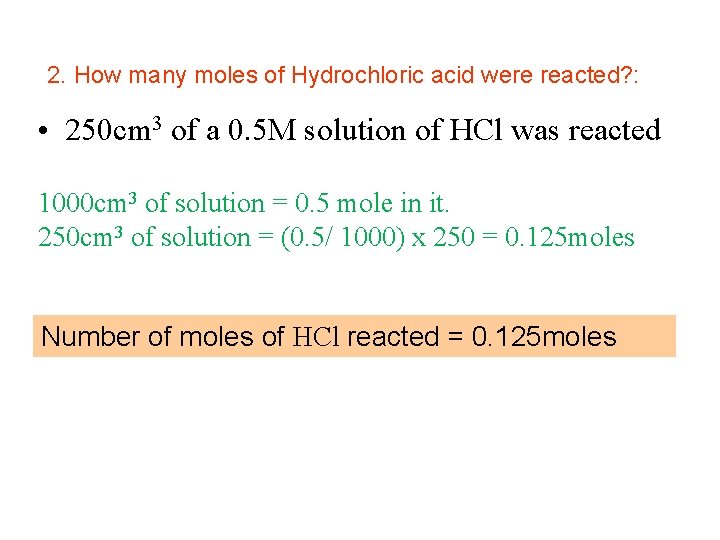
2. How many moles of Hydrochloric acid were reacted? : • 250 cm 3 of a 0. 5 M solution of HCl was reacted 1000 cm 3 of solution = 0. 5 mole in it. 250 cm 3 of solution = (0. 5/ 1000) x 250 = 0. 125 moles Number of moles of HCl reacted = 0. 125 moles

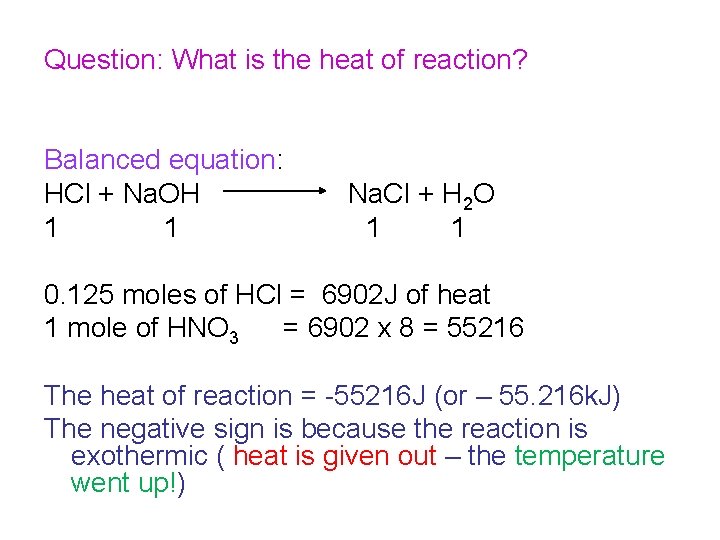
Question: What is the heat of reaction? Balanced equation: HCl + Na. OH 1 1 Na. Cl + H 2 O 1 1 0. 125 moles of HCl = 6902 J of heat 1 mole of HNO 3 = 6902 x 8 = 55216 The heat of reaction = -55216 J (or – 55. 216 k. J) The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

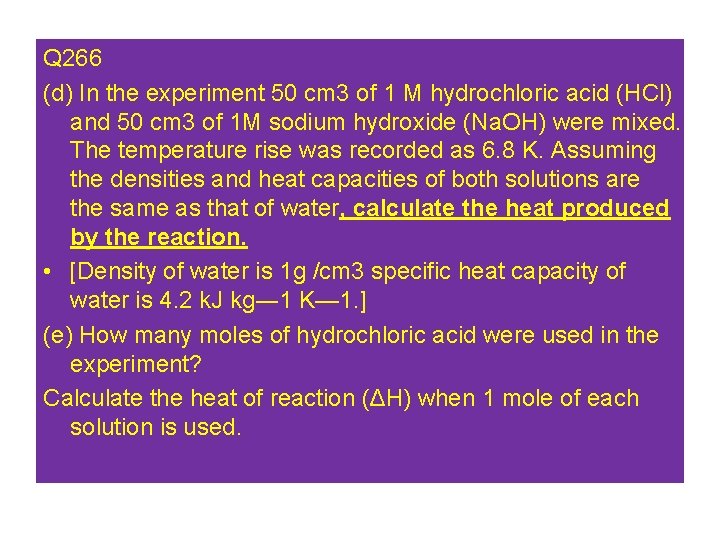
Q 266 (d) In the experiment 50 cm 3 of 1 M hydrochloric acid (HCl) and 50 cm 3 of 1 M sodium hydroxide (Na. OH) were mixed. The temperature rise was recorded as 6. 8 K. Assuming the densities and heat capacities of both solutions are the same as that of water, calculate the heat produced by the reaction. • [Density of water is 1 g /cm 3 specific heat capacity of water is 4. 2 k. J kg― 1 K— 1. ] (e) How many moles of hydrochloric acid were used in the experiment? Calculate the heat of reaction (ΔH) when 1 mole of each solution is used.

1. What is the heat change in the reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Heat change= (. 1 kg) x (4. 2 k. Jkg-1 K-1) x 6. 8 K Heat change = 2. 856 J Specific heat capacity

Q 266 (d) In the experiment 50 cm 3 of 1 M hydrochloric acid (HCl) and 50 cm 3 of 1 M sodium hydroxide (Na. OH) were mixed. The temperature rise was recorded as 6. 8 K. Assuming the densities and heat capacities of both solutions are the same as that of water, calculate the heat produced by the reaction. • [Density of water is 1 g /cm 3 specific heat capacity of water is 4. 2 k. J kg― 1 K— 1. ] (e) How many moles of hydrochloric acid were used in the experiment? Calculate the heat of reaction (ΔH) when 1 mole of each solution is used.

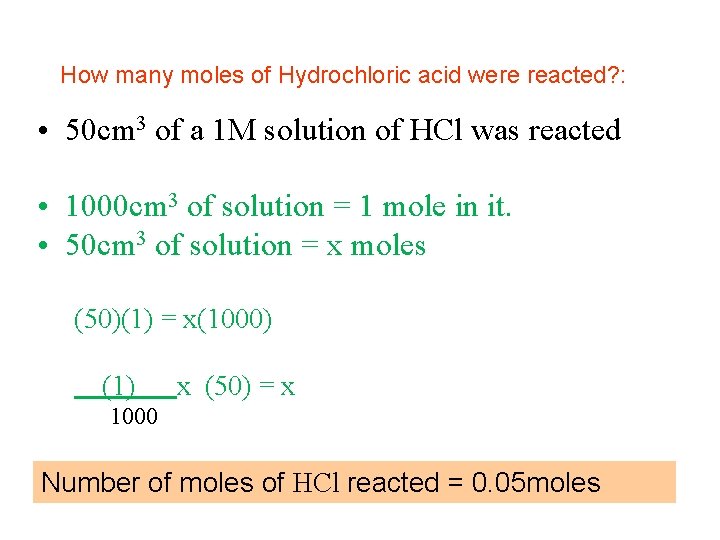
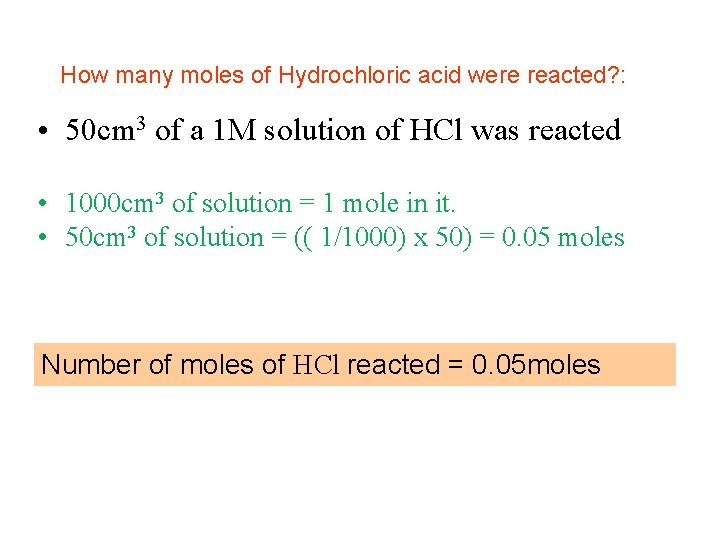
How many moles of Hydrochloric acid were reacted? : • 50 cm 3 of a 1 M solution of HCl was reacted • 1000 cm 3 of solution = 1 mole in it. • 50 cm 3 of solution = x moles (50)(1) = x(1000) (1) x (50) = x 1000 Number of moles of HCl reacted = 0. 05 moles

Q 266 (d) In the experiment 50 cm 3 of 1 M hydrochloric acid (HCl) and 50 cm 3 of 1 M sodium hydroxide (Na. OH) were mixed. The temperature rise was recorded as 6. 8 K. Assuming the densities and heat capacities of both solutions are the same as that of water, calculate the heat produced by the reaction. • [Density of water is 1 g /cm 3 specific heat capacity of water is 4. 2 k. J kg― 1 K— 1. ] (e) How many moles of hydrochloric acid were used in the experiment? Calculate the heat of reaction (ΔH) when 1 mole of each solution is used.

Question: What is the heat of reaction? Balanced equation: HCl + Na. OH 1 1 Na. Cl + H 2 O 1 1 0. 05 moles of HCl = 2. 856 k. J of heat 1 mole of HNO 3 = 2. 856 /20 = 57. 12 J of heat The heat of reaction = -57. 12 k. J The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

• Q 267 • (f) Calculate the number of moles of acid neutralised in this experiment. • In an experiment to measure the heat of reaction for the reaction between sodium hydroxide with hydrochloric acid, a student added 50 cm 3 of 1. 0 M HCl solution to the same volume of 1. 0 M Na. OH solution in a polystyrene foam cup. Taking the total heat capacity of the reaction mixture used in this experiment as 420 J K– 1, calculate the heat released in the experiment if a temperature rise of 6. 7 ºC was recorded. • Hence calculate the heat of reaction for Na. OH + HCl → Na. Cl + H 2 O

How many moles of Hydrochloric acid were reacted? : • 50 cm 3 of a 1 M solution of HCl was reacted • 1000 cm 3 of solution = 1 mole in it. • 50 cm 3 of solution = (( 1/1000) x 50) = 0. 05 moles Number of moles of HCl reacted = 0. 05 moles

• Q 267 • (f) Calculate the number of moles of acid neutralised in this experiment. • In an experiment to measure the heat of reaction for the reaction between sodium hydroxide with hydrochloric acid, a student added 50 cm 3 of 1. 0 M HCl solution to the same volume of 1. 0 M Na. OH solution in a polystyrene foam cup. Taking the total heat capacity of the reaction mixture used in this experiment as 420 J K– 1, calculate the heat released in the experiment if a temperature rise of 6. 7 ºC was recorded. • Hence calculate the heat of reaction for Na. OH + HCl → Na. Cl + H 2 O

1. What is the heat change in the reaction: Heat change = mc ∆ T Temperature rise Mass in kilograms Heat change= (. 1 kg) x (420 Jkg-1 K-1) x 6. 7 o. C Heat change = 281. 4 J Specific heat capacity

Question: What is the heat of reaction? Balanced equation: HCl + Na. OH 1 1 Na. Cl + H 2 O 1 1 0. 05 moles of HCl = 281. 4 J of heat 1 mole of HCl = (281. 4 x 20) = 5628 J of heat The heat of reaction = -5628 J ( - 5. 628 k. J) The negative sign is because the reaction is exothermic ( heat is given out – the temperature went up!)

2009 Q 3, (b) which of the substances was identified by adding silver nitrate solution? KCl ( has chloride ions!), result = white precipitate soluble in ammonia solution (c)what as other reagent added with conc. Sulfuric acid that resulted in a brown ring forming? Iron (II)sulfate solution salt which gave positive result =KNO 3 (d)Test the samples for the phosphate anion = add ammonium molbdate, add a few drops of nitric acid. A yellow precipitate indicates a positive result

(e) Write a balanced equation for one of the equations which gave a white ppt with the Ba. Cl 2 solution. . . Must be the salts with sulfite Na 2 SO 3. 7 H 20 or sulfate ions Na 2 SO 4. 7 H 2 O What was observed when student added dilute HCl? White ppt dissolves in the sulfite Na 2 SO 3. 7 H 20, But doesn't in the sulfate Na 2 SO 4. 7 H 2 O (f)Suggest a way to confirm the presence of the last salt Hydrogen carbonate salt Na. HCO 3 remains. Add dilute HCl, carbon dioxide gas should be released( limewater will go cloudy), add magnesium sulfate to a fresh sample. No white precipitate should be formed

Bond energy

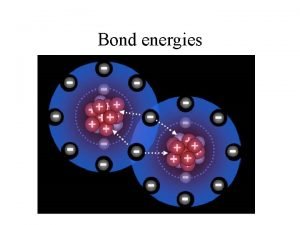
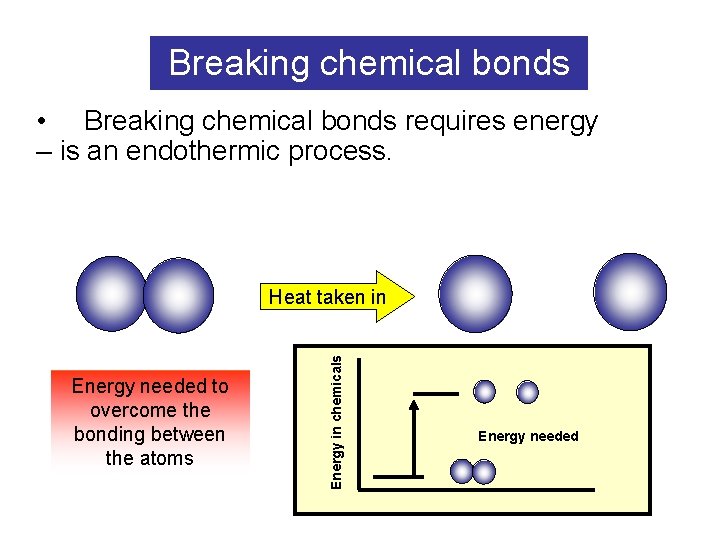
Breaking chemical bonds • Breaking chemical bonds requires energy – is an endothermic process. Energy needed to overcome the bonding between the atoms Energy in chemicals Heat taken in Energy needed

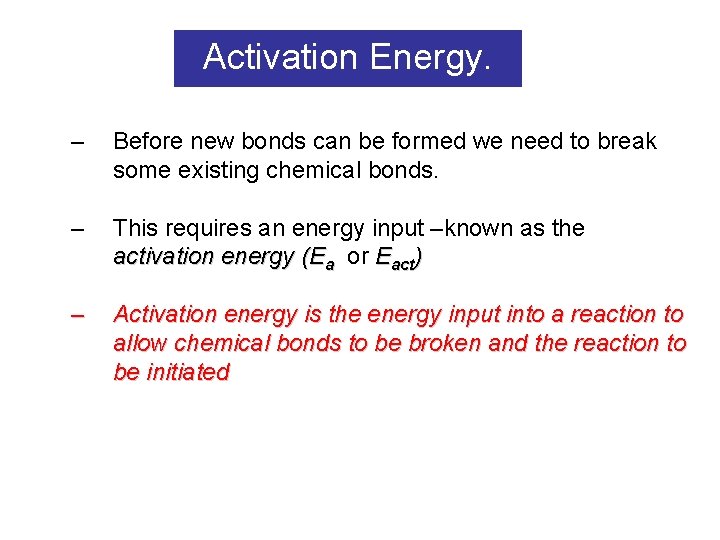
Activation Energy. – Before new bonds can be formed we need to break some existing chemical bonds. – This requires an energy input –known as the activation energy (Ea or Eact) – Activation energy is the energy input into a reaction to allow chemical bonds to be broken and the reaction to be initiated

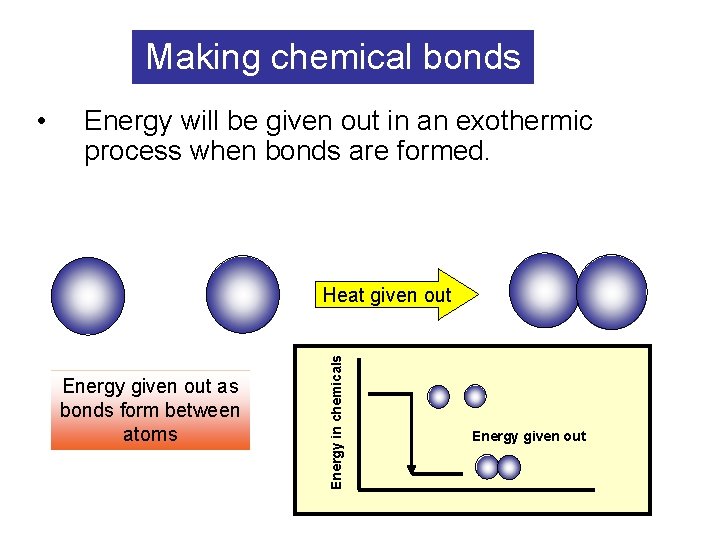
Making chemical bonds Energy will be given out in an exothermic process when bonds are formed. Heat given out Energy given out as bonds form between atoms Energy in chemicals • Energy given out

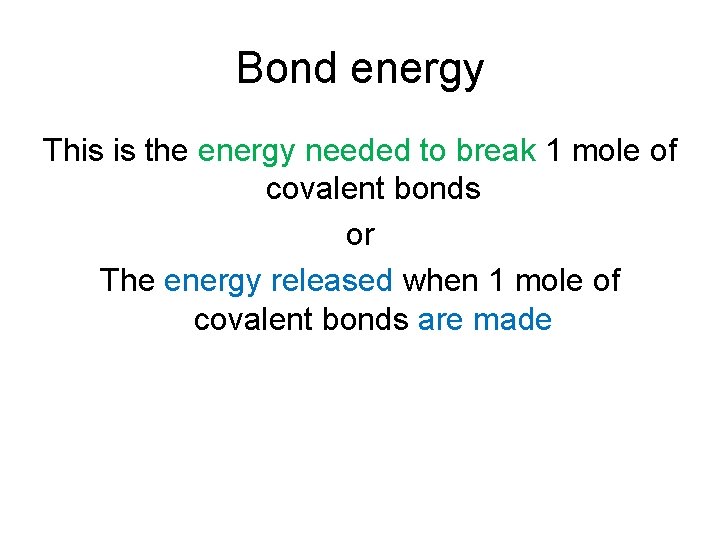
Bond energy This is the energy needed to break 1 mole of covalent bonds or The energy released when 1 mole of covalent bonds are made

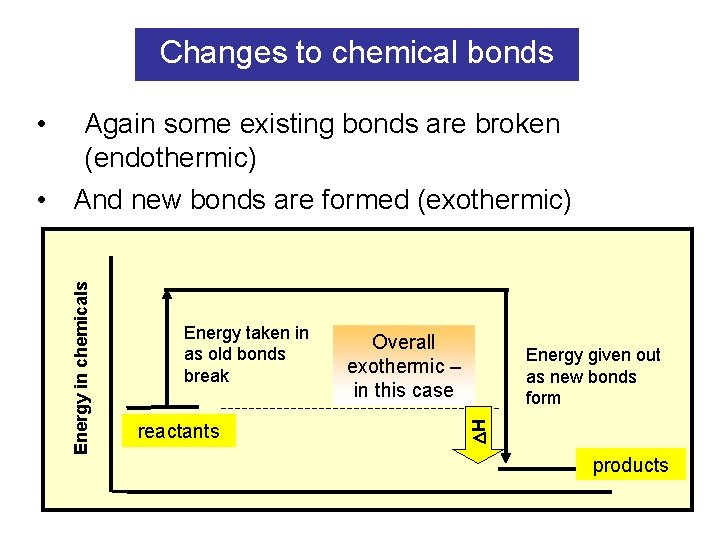
Changes to chemical bonds • Again some existing bonds are broken (endothermic) Energy taken in as old bonds break reactants Overall exothermic – in this case Energy given out as new bonds form H Energy in chemicals • And new bonds are formed (exothermic) products

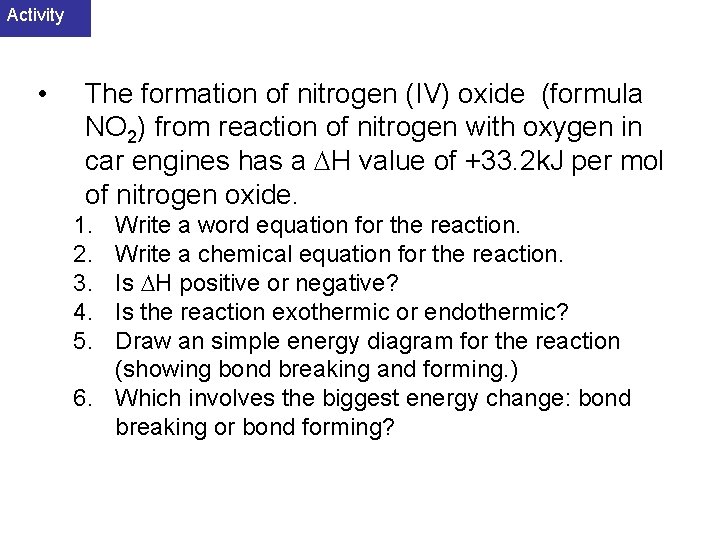
Activity • The formation of nitrogen (IV) oxide (formula NO 2) from reaction of nitrogen with oxygen in car engines has a H value of +33. 2 k. J per mol of nitrogen oxide. 1. 2. 3. 4. 5. Write a word equation for the reaction. Write a chemical equation for the reaction. Is H positive or negative? Is the reaction exothermic or endothermic? Draw an simple energy diagram for the reaction (showing bond breaking and forming. ) 6. Which involves the biggest energy change: bond breaking or bond forming?

Answer 1. 2. 3. 4. 5. 6. Nitrogen + oxygen nitrogen(IV)oxide N 2 + 2 O 2 2 NO 2. H positive (+33. 2 k. J/mol). The reaction is endothermic. Energy diagram Bond breaking involves the biggest energy change.

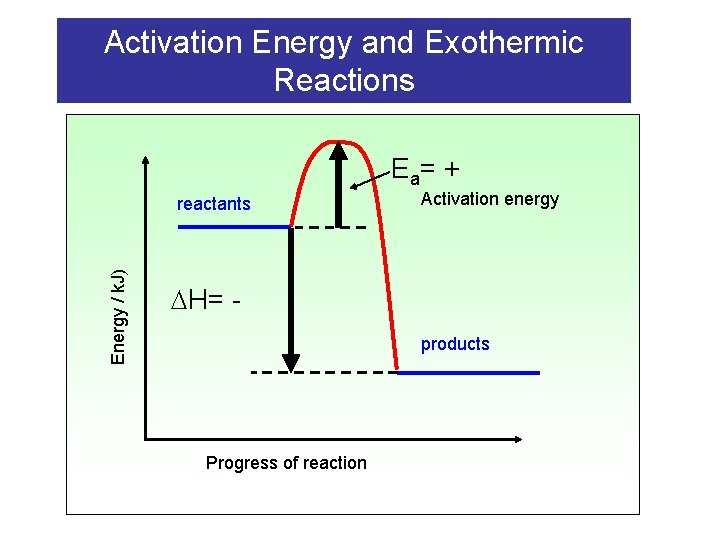
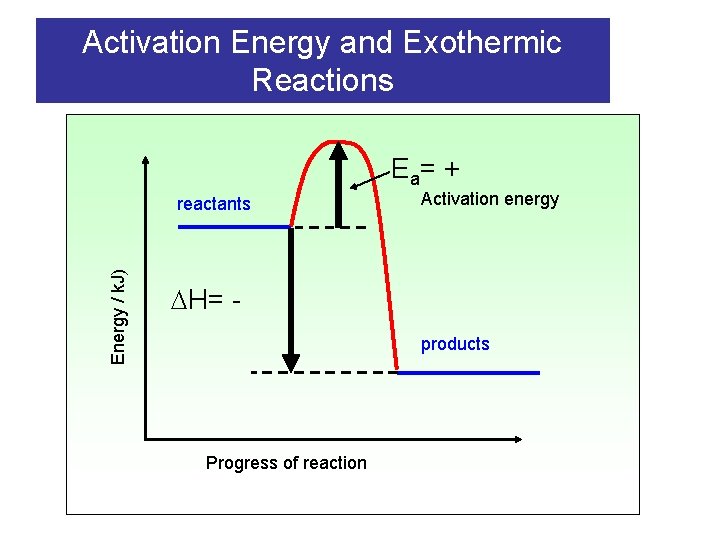
Activation Energy and Exothermic Reactions E a= + Energy / k. J) reactants Activation energy H= products Progress of reaction

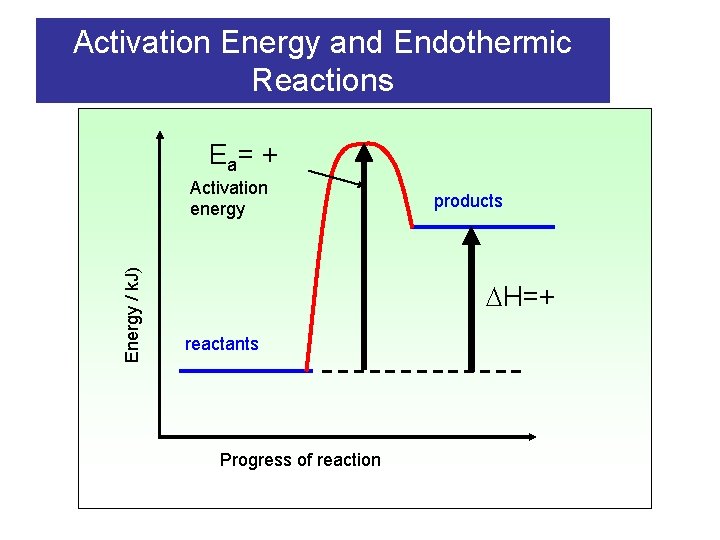
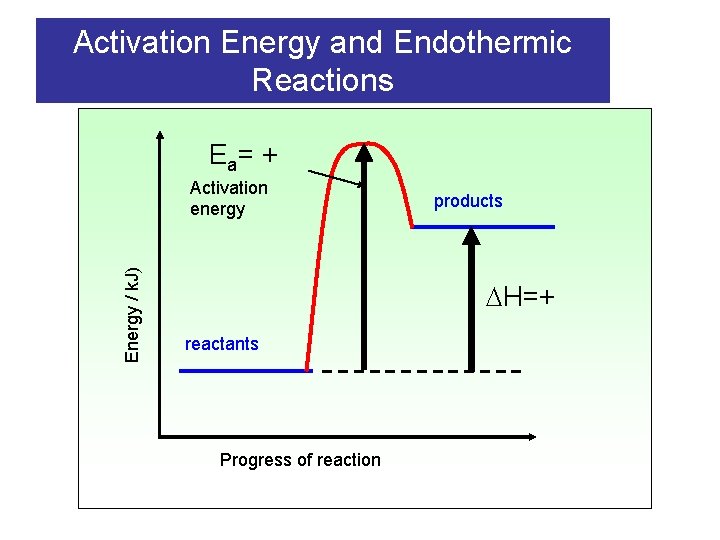
Activation Energy and Endothermic Reactions E a= + Energy / k. J) Activation energy products H=+ reactants Progress of reaction

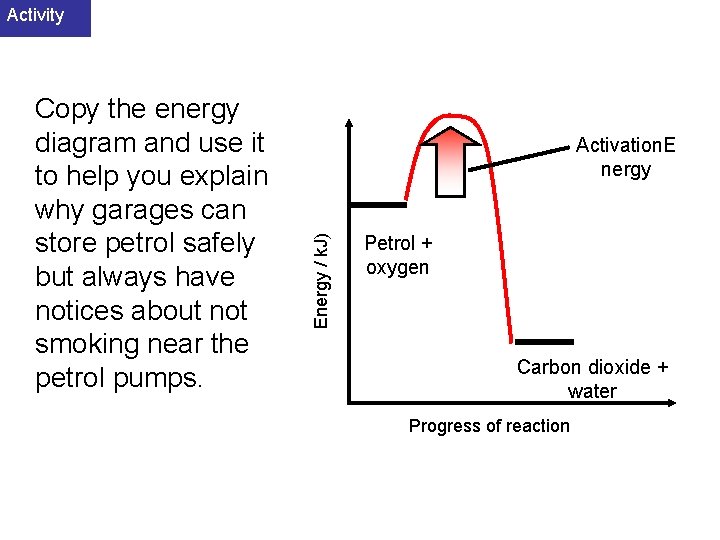
Activity Activation. E nergy Energy / k. J) Copy the energy diagram and use it to help you explain why garages can store petrol safely but always have notices about not smoking near the petrol pumps. Petrol + oxygen Carbon dioxide + water Progress of reaction

Check your learning • What is an exothermic reaction? • What is an endothermic reaction? • Define heat of reaction

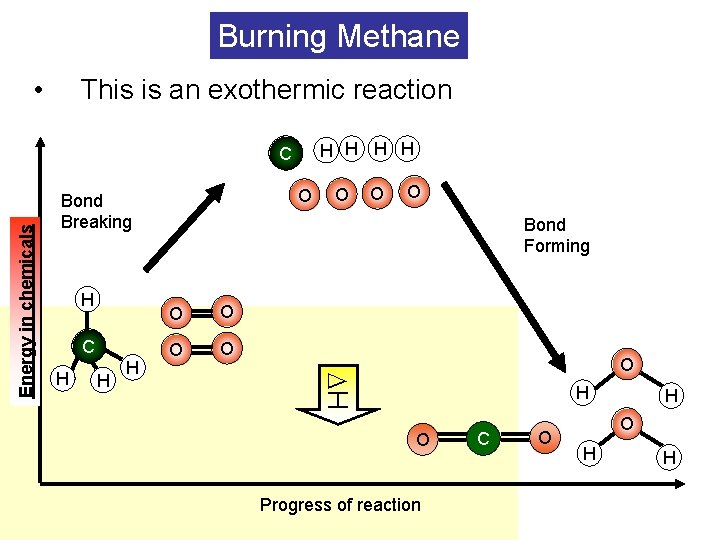
Burning Methane • This is an exothermic reaction H H O Bond Breaking H C H H H O O O Bond Forming O O O H Energy in chemicals C H O Progress of reaction C O H H

Activation Energy and Exothermic Reactions E a= + Energy / k. J) reactants Activation energy H= products Progress of reaction

Activation Energy and Endothermic Reactions E a= + Energy / k. J) Activation energy products H=+ reactants Progress of reaction

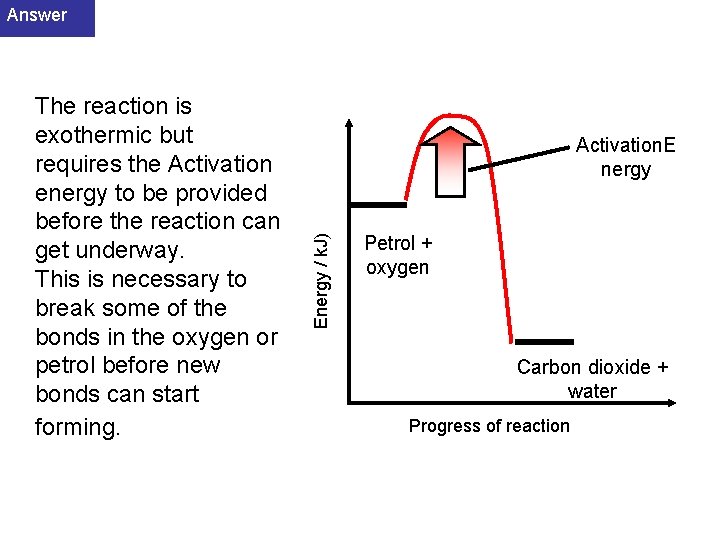
Answer Activation. E nergy Energy / k. J) The reaction is exothermic but requires the Activation energy to be provided before the reaction can get underway. This is necessary to break some of the bonds in the oxygen or petrol before new bonds can start forming. Petrol + oxygen Carbon dioxide + water Progress of reaction

Learning objectives • Definitions: Heat of combustion, Heat of Formation, Hesses Law • Doing calculations incvolving Hesses Law

Homework Thermochemistry Ol 2007 Q 3 Revision Ch 4, 5 HL 2007 Q 4(f), 2004 Q 4 j, 2003 Q 5(c) OL 2007 Q 4 H, Q 5 a, c, Q 11 a

Heat of combustion • The heat change that occurs when 1 mole of substance is burned in excess oxygen Uses of heat generated from burning substances Transportation Generating electricity


A bomb calorimeter • Use : measuring the energy contents of fuels or foods

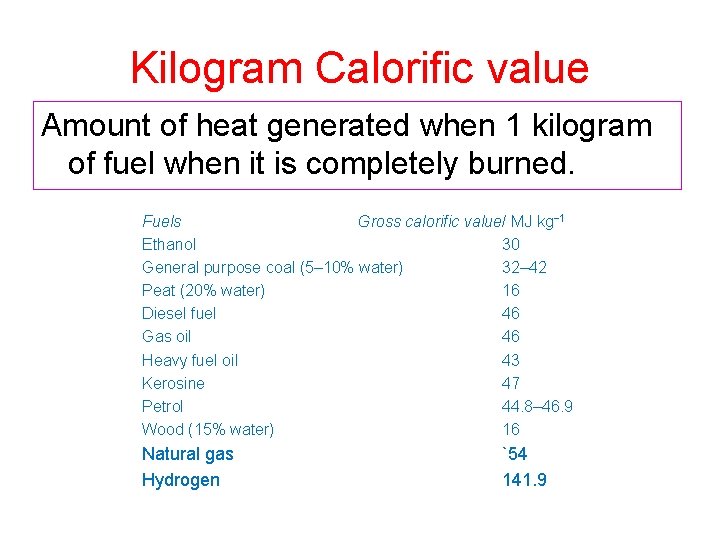
Kilogram Calorific value Amount of heat generated when 1 kilogram of fuel when it is completely burned. Fuels Gross calorific value/ MJ kg− 1 Ethanol 30 General purpose coal (5– 10% water) 32– 42 Peat (20% water) 16 Diesel fuel 46 Gas oil 46 Heavy fuel oil 43 Kerosine 47 Petrol 44. 8– 46. 9 Wood (15% water) 16 Natural gas Hydrogen `54 141. 9

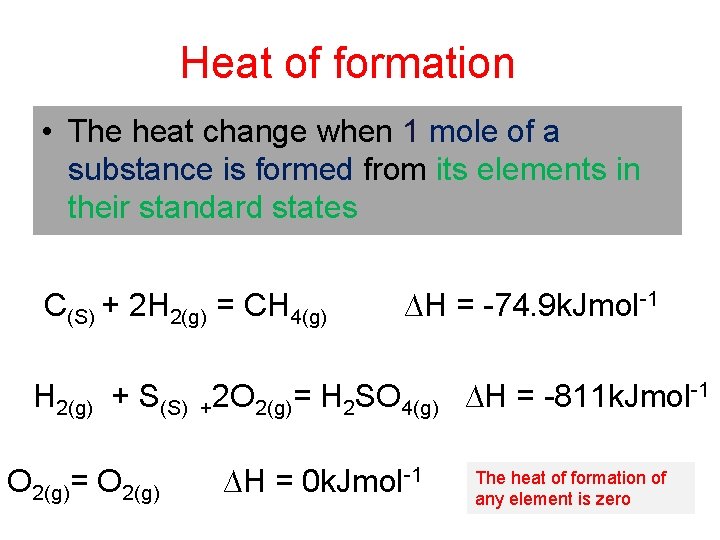
Heat of formation • The heat change when 1 mole of a substance is formed from its elements in their standard states C(S) + 2 H 2(g) = CH 4(g) ∆H = -74. 9 k. Jmol-1 H 2(g) + S(S) +2 O 2(g)= H 2 SO 4(g) ∆H = -811 k. Jmol-1 O 2(g)= O 2(g) ∆H = 0 k. Jmol-1 The heat of formation of any element is zero

Check your learning. . • • • Define each of the following… Heat of reaction Heat of combustion Kilogram Calorific Value Heat of formation Hesses Law

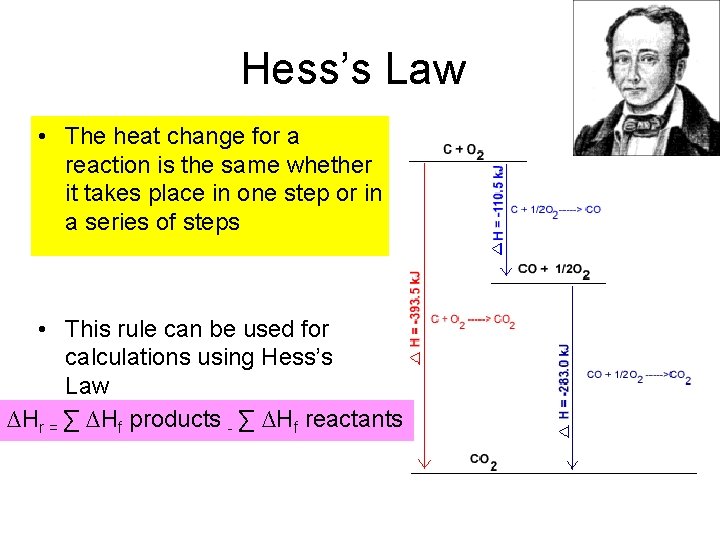
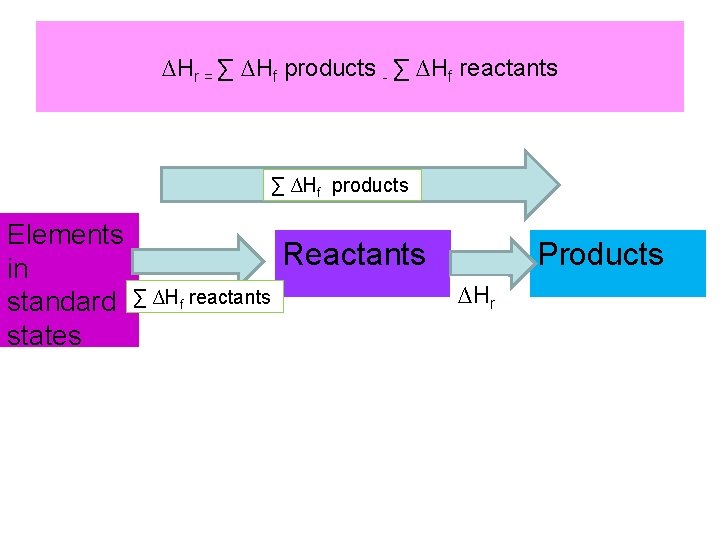
Hess’s Law • The heat change for a reaction is the same whether it takes place in one step or in a series of steps • This rule can be used for calculations using Hess’s Law ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants

∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants ∑ ∆Hf products Elements in standard states Reactants ∑ ∆Hf reactants Products ∆Hr

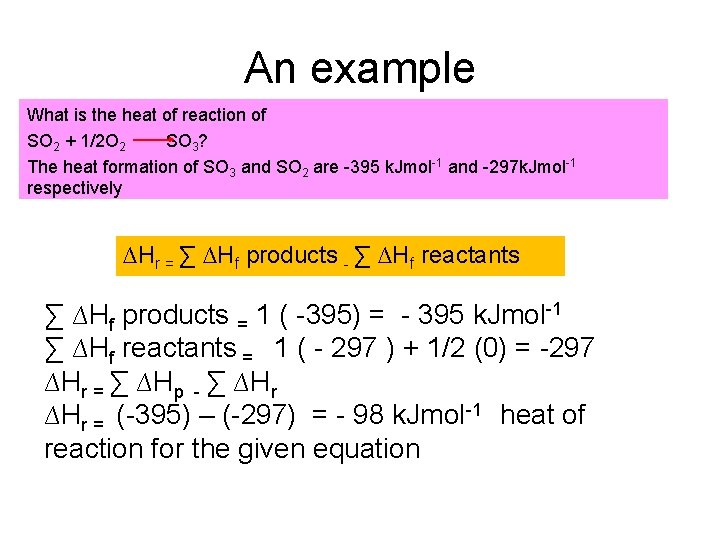
An example What is the heat of reaction of SO 2 + 1/2 O 2 SO 3? The heat formation of SO 3 and SO 2 are -395 k. Jmol-1 and -297 k. Jmol-1 respectively ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants ∑ ∆Hf products = 1 ( -395) = - 395 k. Jmol-1 ∑ ∆Hf reactants = 1 ( - 297 ) + 1/2 (0) = -297 ∆Hr = ∑ ∆Hp - ∑ ∆Hr = (-395) – (-297) = - 98 k. Jmol-1 heat of reaction for the given equation

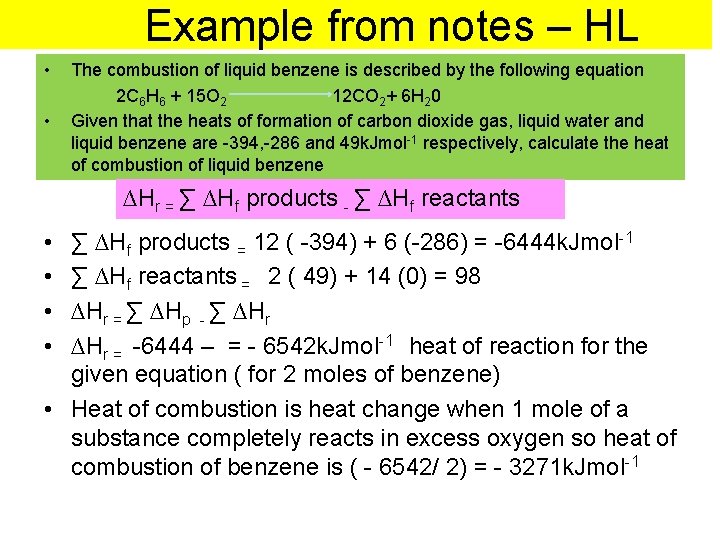
Example from notes – HL • • The combustion of liquid benzene is described by the following equation 2 C 6 H 6 + 15 O 2 12 CO 2+ 6 H 20 Given that the heats of formation of carbon dioxide gas, liquid water and liquid benzene are -394, -286 and 49 k. Jmol-1 respectively, calculate the heat of combustion of liquid benzene ∆Hr = ∑ ∆H∆H ∆Hf rreactants f products r = ∑ ∆Hp- ∑ - ∑∆H • • ∑ ∆Hf products = 12 ( -394) + 6 (-286) = -6444 k. Jmol-1 ∑ ∆Hf reactants = 2 ( 49) + 14 (0) = 98 ∆Hr = ∑ ∆Hp - ∑ ∆Hr = -6444 – = - 6542 k. Jmol-1 heat of reaction for the given equation ( for 2 moles of benzene) • Heat of combustion is heat change when 1 mole of a substance completely reacts in excess oxygen so heat of combustion of benzene is ( - 6542/ 2) = - 3271 k. Jmol-1

Try now… • Hl 2006 Q 6 c, 2008 Q 6 b

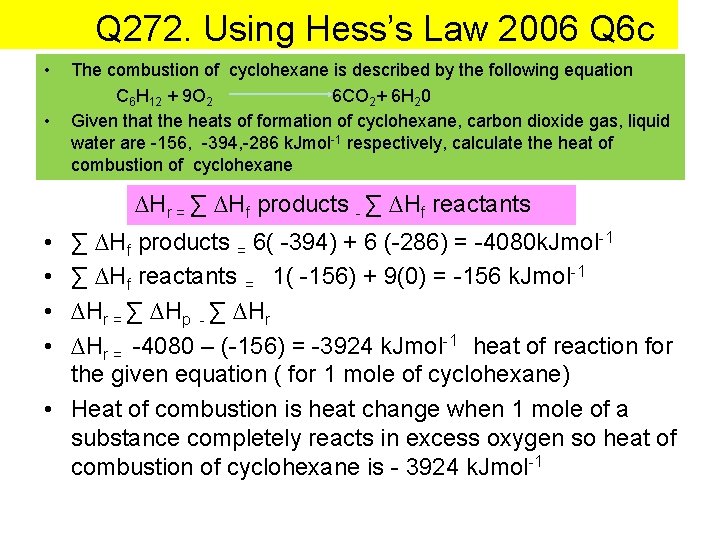
Q 272. Using Hess’s Law 2006 Q 6 c • • The combustion of cyclohexane is described by the following equation C 6 H 12 + 9 O 2 6 CO 2+ 6 H 20 Given that the heats of formation of cyclohexane, carbon dioxide gas, liquid water are -156, -394, -286 k. Jmol-1 respectively, calculate the heat of combustion of cyclohexane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∑ ∆Hf products = 6( -394) + 6 (-286) = -4080 k. Jmol-1 ∑ ∆Hf reactants = 1( -156) + 9(0) = -156 k. Jmol-1 ∆Hr = ∑ ∆Hp - ∑ ∆Hr = -4080 – (-156) = -3924 k. Jmol-1 heat of reaction for the given equation ( for 1 mole of cyclohexane) • Heat of combustion is heat change when 1 mole of a substance completely reacts in excess oxygen so heat of combustion of cyclohexane is - 3924 k. Jmol-1

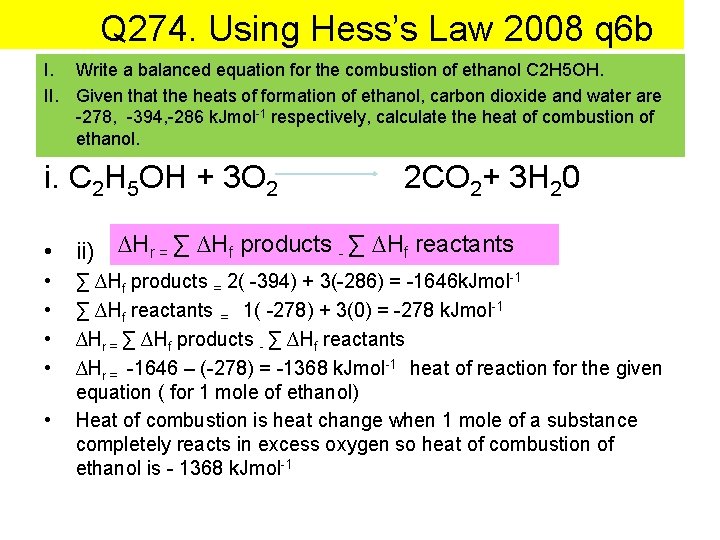
Q 274. Using Hess’s Law 2008 q 6 b I. Write a balanced equation for the combustion of ethanol C 2 H 5 OH. II. Given that the heats of formation of ethanol, carbon dioxide and water are -278, -394, -286 k. Jmol-1 respectively, calculate the heat of combustion of ethanol. i. C 2 H 5 OH + 3 O 2 2 CO 2+ 3 H 20 • ii) ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • • ∑ ∆Hf products = 2( -394) + 3(-286) = -1646 k. Jmol-1 ∑ ∆Hf reactants = 1( -278) + 3(0) = -278 k. Jmol-1 ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants ∆Hr = -1646 – (-278) = -1368 k. Jmol-1 heat of reaction for the given equation ( for 1 mole of ethanol) Heat of combustion is heat change when 1 mole of a substance completely reacts in excess oxygen so heat of combustion of ethanol is - 1368 k. Jmol-1

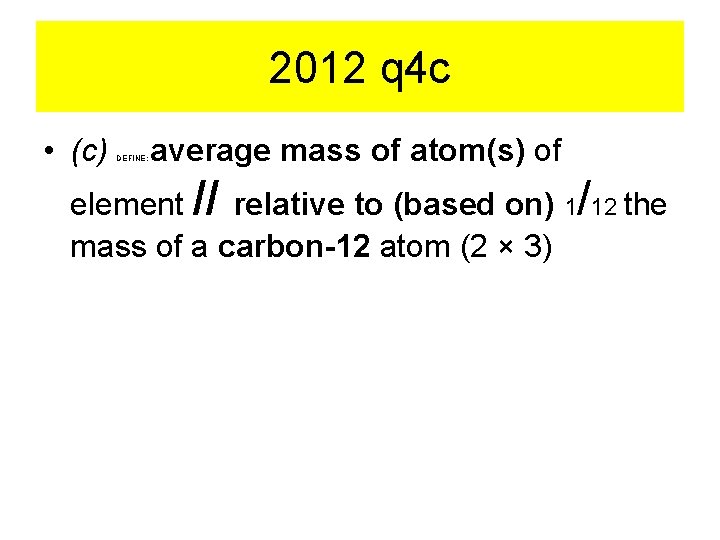
2012 q 4 c • (c) DEFINE: average mass of atom(s) of element // relative to (based on) 1/12 the mass of a carbon-12 atom (2 × 3)

2011 q 4 a

2011 Q 5 a, b


2011 Q 5 c

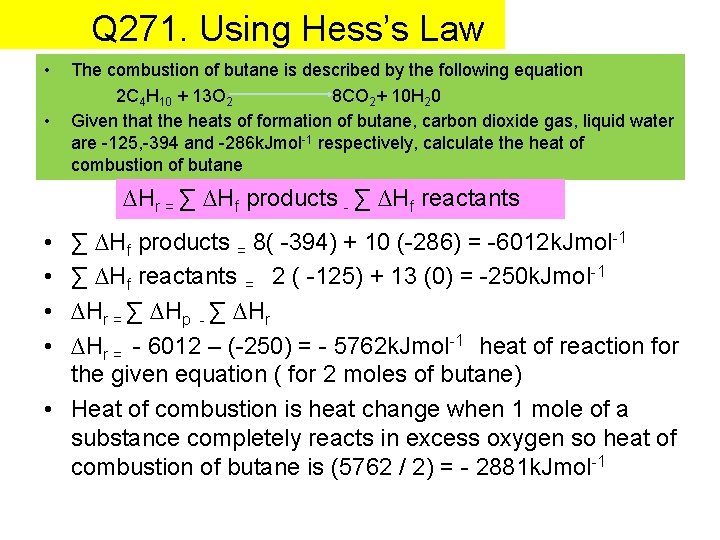
Q 271. Using Hess’s Law • • The combustion of butane is described by the following equation 2 C 4 H 10 + 13 O 2 8 CO 2+ 10 H 20 Given that the heats of formation of butane, carbon dioxide gas, liquid water are -125, -394 and -286 k. Jmol-1 respectively, calculate the heat of combustion of butane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∑ ∆Hf products = 8( -394) + 10 (-286) = -6012 k. Jmol-1 ∑ ∆Hf reactants = 2 ( -125) + 13 (0) = -250 k. Jmol-1 ∆Hr = ∑ ∆Hp - ∑ ∆Hr = - 6012 – (-250) = - 5762 k. Jmol-1 heat of reaction for the given equation ( for 2 moles of butane) • Heat of combustion is heat change when 1 mole of a substance completely reacts in excess oxygen so heat of combustion of butane is (5762 / 2) = - 2881 k. Jmol-1

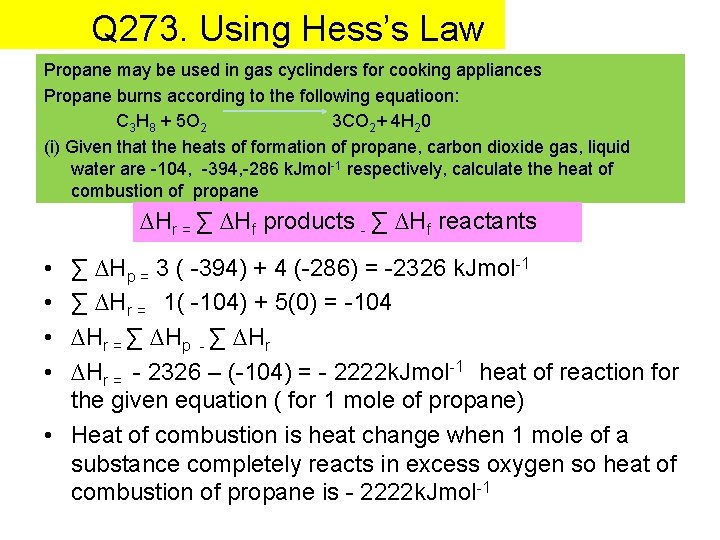
Q 273. Using Hess’s Law Propane may be used in gas cyclinders for cooking appliances Propane burns according to the following equatioon: C 3 H 8 + 5 O 2 3 CO 2+ 4 H 20 (i) Given that the heats of formation of propane, carbon dioxide gas, liquid water are -104, -394, -286 k. Jmol-1 respectively, calculate the heat of combustion of propane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∑ ∆Hp = 3 ( -394) + 4 (-286) = -2326 k. Jmol-1 ∑ ∆Hr = 1( -104) + 5(0) = -104 ∆Hr = ∑ ∆Hp - ∑ ∆Hr = - 2326 – (-104) = - 2222 k. Jmol-1 heat of reaction for the given equation ( for 1 mole of propane) • Heat of combustion is heat change when 1 mole of a substance completely reacts in excess oxygen so heat of combustion of propane is - 2222 k. Jmol-1

• If 500 k. J of energy are needed to boil a kettle of water what mass of propane gas must be burned to generate this mass of heat? Give your answer to the nearest gram Find number of moles needed Find the number of grams needed x moles of propane = 500 k. J of heat released when combusted 1 mole of propane = 2222 Kj of heat released when combusted 500 x 1 = x 2222 0. 22502 = x 0. 225 = number of moles of propane needed to release 500 k. J of heat when combusted

• If 500 k. J of energy are needed to boil a kettle of water what mass of propane gas must be burned to generate this mass of heat? Give your answer to the nearest gram Find number of moles needed Find the number of grams needed X RMM 0. 225 moles of propane , how many grams? (44)(0. 225) = 9. 901 Answer : 9. 901 g of propane would generate 5000 Kj of heat

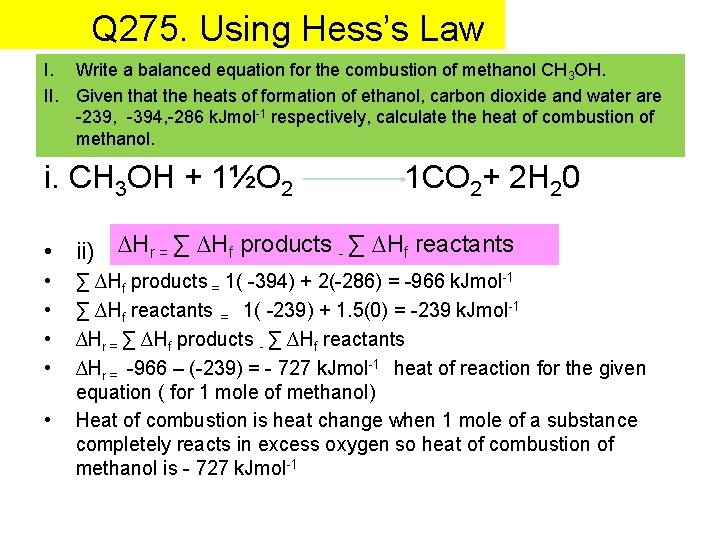
Q 275. Using Hess’s Law I. Write a balanced equation for the combustion of methanol CH 3 OH. II. Given that the heats of formation of ethanol, carbon dioxide and water are -239, -394, -286 k. Jmol-1 respectively, calculate the heat of combustion of methanol. i. CH 3 OH + 1½O 2 1 CO 2+ 2 H 20 • ii) ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • • ∑ ∆Hf products = 1( -394) + 2(-286) = -966 k. Jmol-1 ∑ ∆Hf reactants = 1( -239) + 1. 5(0) = -239 k. Jmol-1 ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants ∆Hr = -966 – (-239) = - 727 k. Jmol-1 heat of reaction for the given equation ( for 1 mole of methanol) Heat of combustion is heat change when 1 mole of a substance completely reacts in excess oxygen so heat of combustion of methanol is - 727 k. Jmol-1

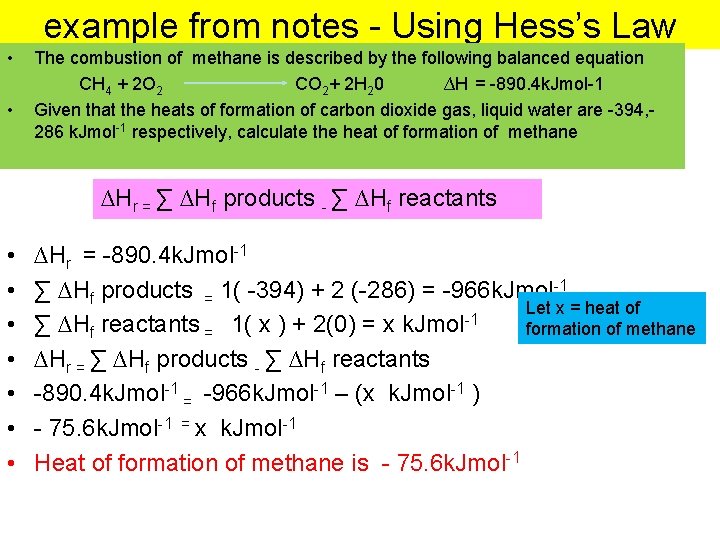
example from notes - Using Hess’s Law • • The combustion of methane is described by the following balanced equation CH 4 + 2 O 2 CO 2+ 2 H 20 ∆H = -890. 4 k. Jmol-1 Given that the heats of formation of carbon dioxide gas, liquid water are -394, 286 k. Jmol-1 respectively, calculate the heat of formation of methane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∆Hr = -890. 4 k. Jmol-1 ∑ ∆Hf products = 1( -394) + 2 (-286) = -966 k. Jmol-1 Let x = heat of -1 ∑ ∆Hf reactants = 1( x ) + 2(0) = x k. Jmol formation of methane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants -890. 4 k. Jmol-1 = -966 k. Jmol-1 – (x k. Jmol-1 ) - 75. 6 k. Jmol-1 = x k. Jmol-1 Heat of formation of methane is - 75. 6 k. Jmol-1

• • Q 277) Using Hess’s Law The combustion of ethyne is described by the following balanced equation C 2 H 2 + 2½O 2 2 CO 2+ H 20 ∆H = -1299 k. Jmol-1 Given that the heats of formation of carbon dioxide gas, liquid water are -394, 286 k. Jmol-1 respectively, calculate the heat of formation of ethyne ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∆Hr = -1299 k. Jmol-1 ∑ ∆Hf products = 2( -394) + 1(-286) = -1074 k. Jmol-1 ∑ ∆Hf reactants = 1( x ) + 2½ (0) = x k. Jmol-1 Let x = heat of formation of ethyne ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants -1299 k. Jmol-1 = -1074 k. Jmol-1 – (x k. Jmol-1 ) - 225 k. Jmol-1 =- x k. Jmol-1 Heat of formation of ethyne is 225 k. Jmol-1

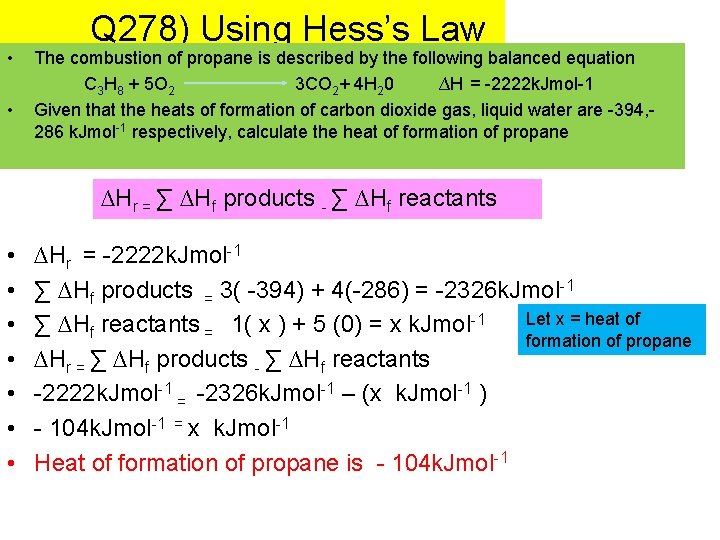
• • Q 278) Using Hess’s Law The combustion of propane is described by the following balanced equation C 3 H 8 + 5 O 2 3 CO 2+ 4 H 20 ∆H = -2222 k. Jmol-1 Given that the heats of formation of carbon dioxide gas, liquid water are -394, 286 k. Jmol-1 respectively, calculate the heat of formation of propane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∆Hr = -2222 k. Jmol-1 ∑ ∆Hf products = 3( -394) + 4(-286) = -2326 k. Jmol-1 Let x = heat of ∑ ∆Hf reactants = 1( x ) + 5 (0) = x k. Jmol-1 formation of propane ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants -2222 k. Jmol-1 = -2326 k. Jmol-1 – (x k. Jmol-1 ) - 104 k. Jmol-1 = x k. Jmol-1 Heat of formation of propane is - 104 k. Jmol-1

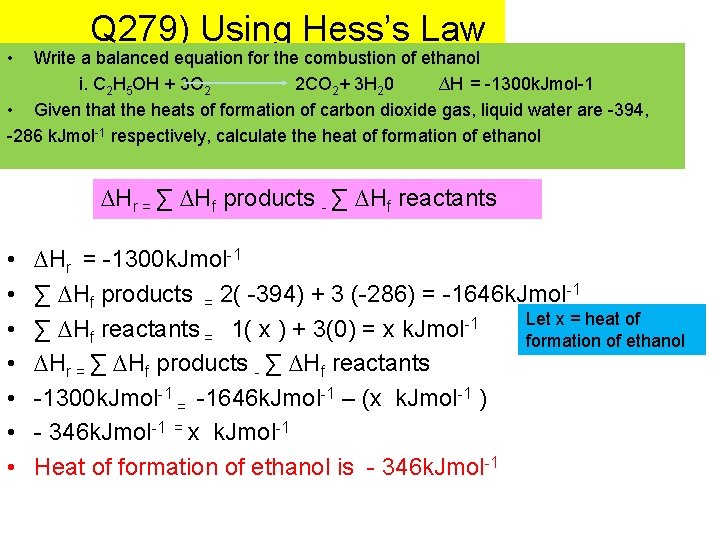
• Q 279) Using Hess’s Law Write a balanced equation for the combustion of ethanol i. C 2 H 5 OH + 3 O 2 2 CO 2+ 3 H 20 ∆H = -1300 k. Jmol-1 • Given that the heats of formation of carbon dioxide gas, liquid water are -394, -286 k. Jmol-1 respectively, calculate the heat of formation of ethanol ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants • • ∆Hr = -1300 k. Jmol-1 ∑ ∆Hf products = 2( -394) + 3 (-286) = -1646 k. Jmol-1 Let x = heat of ∑ ∆Hf reactants = 1( x ) + 3(0) = x k. Jmol-1 formation of ethanol ∆Hr = ∑ ∆Hf products - ∑ ∆Hf reactants -1300 k. Jmol-1 = -1646 k. Jmol-1 – (x k. Jmol-1 ) - 346 k. Jmol-1 = x k. Jmol-1 Heat of formation of ethanol is - 346 k. Jmol-1

• Thermochemistry HL 2015 Q 6 c, 2009 Q 6 e OL 2015 q 10 b Homework Revision – ch 6, 7 Hl 2015 Q 11 a Ol 2015 Q 4 c, q 5 Test next Monday part 1 ch 1 -7, part 2 thermochemistry ch 24
 Endo exo thermic
Endo exo thermic Define exothermic reaction
Define exothermic reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Endothermic vs exothermic
Endothermic vs exothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Heating cooling curve
Heating cooling curve Exo vs endothermic
Exo vs endothermic Examples of ectotherms
Examples of ectotherms Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic Ins
Ins Exothermic or endothermic
Exothermic or endothermic Exothermic vs endothermic
Exothermic vs endothermic Freezing melting evaporation condensation sublimation
Freezing melting evaporation condensation sublimation Is bond breaking endothermic or exothermic
Is bond breaking endothermic or exothermic Is the combustion of propane endothermic or exothermic
Is the combustion of propane endothermic or exothermic Endo vs exo graphs
Endo vs exo graphs Is a firecracker exploding endothermic or exothermic
Is a firecracker exploding endothermic or exothermic Is transpiration endothermic or exothermic
Is transpiration endothermic or exothermic Is baking bread endothermic or exothermic
Is baking bread endothermic or exothermic Phase change diagram endothermic exothermic
Phase change diagram endothermic exothermic Types of reactions
Types of reactions Teen movies genre
Teen movies genre Endothermic reaction examples
Endothermic reaction examples Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations 5 examples of redox reaction
5 examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Reactants and products
Reactants and products Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes I intro
I intro Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reaction of copper and percent yield
Chemical reaction of copper and percent yield What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Endothermic and endergonic
Endothermic and endergonic Governing marriage laws and conducting elections involve
Governing marriage laws and conducting elections involve Spermatogenesis and oogenesis both involve
Spermatogenesis and oogenesis both involve Stoichiometry mole island diagram
Stoichiometry mole island diagram Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Immunoelectrophoresis
Immunoelectrophoresis Predicting products of chemical reactions
Predicting products of chemical reactions Chemistry predicting products
Chemistry predicting products Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Four types of chemical reactions
Four types of chemical reactions Biology-roots.com
Biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Five chemical changes
Five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions
Chapter 9 chemical reactions Equilibrium of chemical reactions
Equilibrium of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide 5 chemical reactions
5 chemical reactions 5 type of reactions
5 type of reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Functional isomers of carboxylic acid
Functional isomers of carboxylic acid Summary of chemical reactions
Summary of chemical reactions Chemical reaction of bread
Chemical reaction of bread Solvent in chemical reactions
Solvent in chemical reactions Fatoumata dembele chef
Fatoumata dembele chef Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of chemical reaction
Indications of chemical reaction Describing chemical reactions
Describing chemical reactions Rules of chemical reactions
Rules of chemical reactions Rearrangement of atoms
Rearrangement of atoms Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations Classification of chemical reactions worksheet
Classification of chemical reactions worksheet Combustion reaction
Combustion reaction Laser beam welding (lbw)
Laser beam welding (lbw) Types of reactions grade 11
Types of reactions grade 11 A balanced chemical reaction obeys the law of
A balanced chemical reaction obeys the law of Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key