Exercises 2 Exercise 2 1 Exercise 2 1


































- Slides: 56

Exercises 2

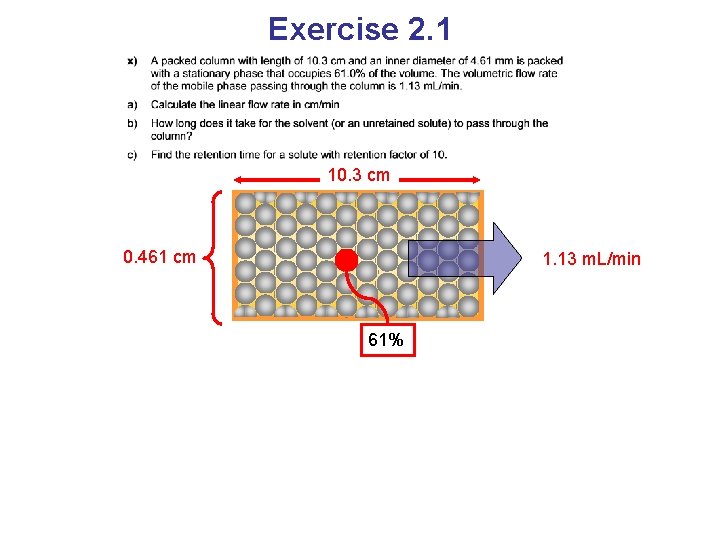
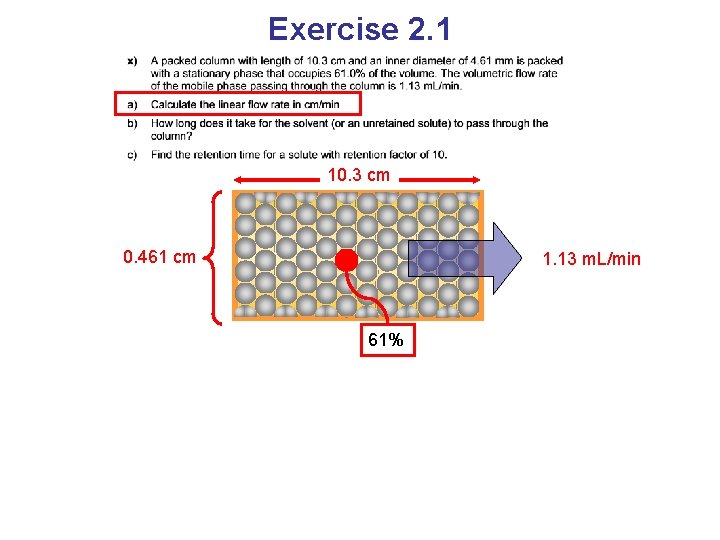
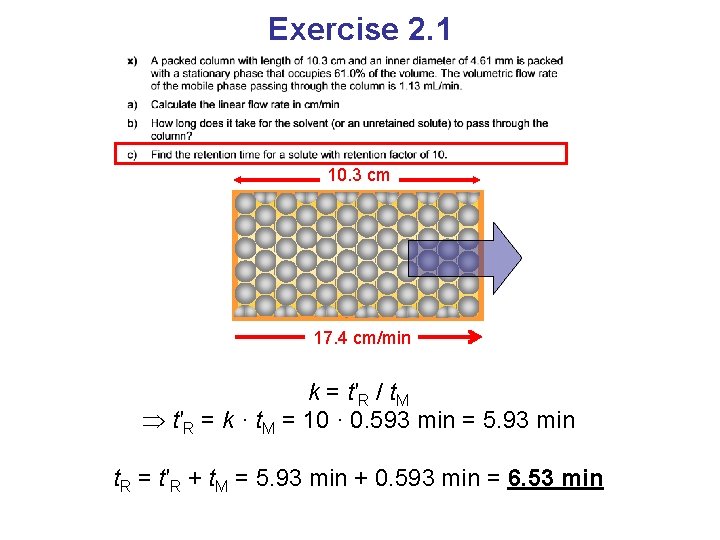
Exercise 2. 1

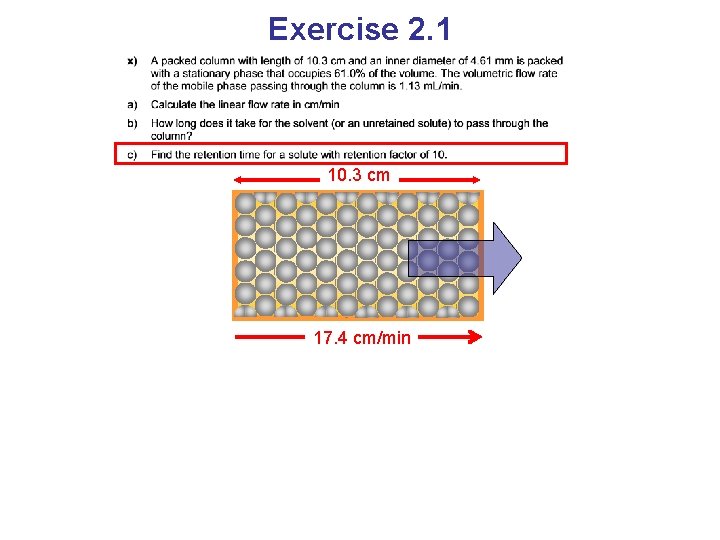
Exercise 2. 1 10. 3 cm 0. 461 cm 1. 13 m. L/min 61%

Exercise 2. 1 10. 3 cm 0. 461 cm 1. 13 m. L/min 61%

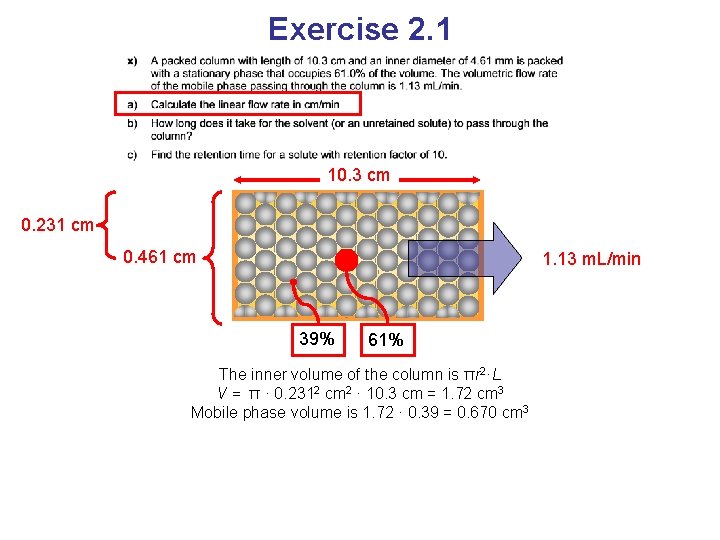
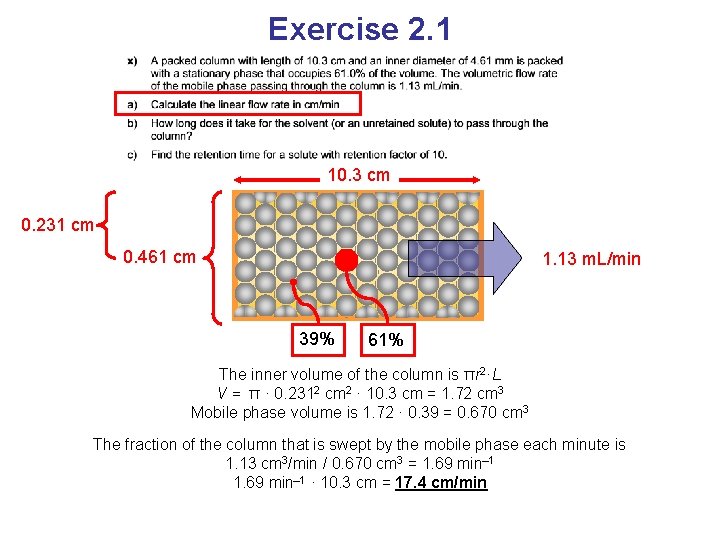
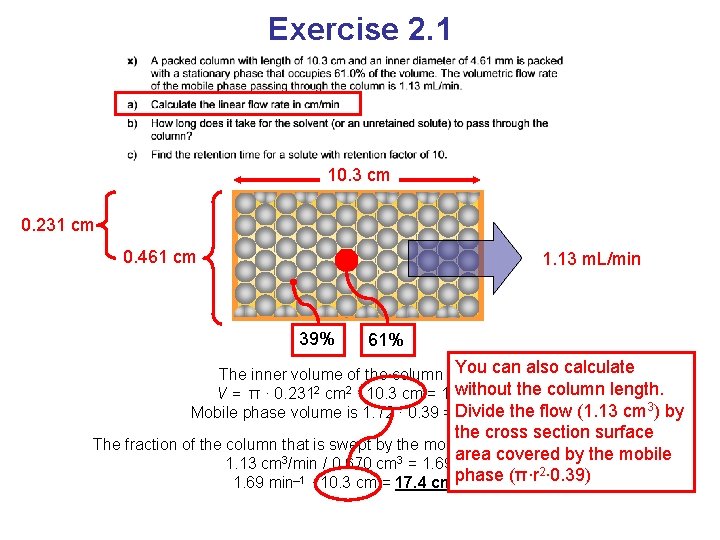
Exercise 2. 1 10. 3 cm 0. 231 cm 0. 461 cm 1. 13 m. L/min 39% 61% The inner volume of the column is πr 2·L V = π · 0. 2312 cm 2 · 10. 3 cm = 1. 72 cm 3 Mobile phase volume is 1. 72 · 0. 39 = 0. 670 cm 3

Exercise 2. 1 10. 3 cm 0. 231 cm 0. 461 cm 1. 13 m. L/min 39% 61% The inner volume of the column is πr 2·L V = π · 0. 2312 cm 2 · 10. 3 cm = 1. 72 cm 3 Mobile phase volume is 1. 72 · 0. 39 = 0. 670 cm 3 The fraction of the column that is swept by the mobile phase each minute is 1. 13 cm 3/min / 0. 670 cm 3 = 1. 69 min– 1 · 10. 3 cm = 17. 4 cm/min

Exercise 2. 1 10. 3 cm 0. 231 cm 0. 461 cm 1. 13 m. L/min 39% 61% can also calculate The inner volume of the column is. You πr 2·L without V = π · 0. 2312 cm 2 · 10. 3 cm = 1. 72 cm 3 the column length. 3 flow (1. 13 cm 3) by the Mobile phase volume is 1. 72 · 0. 39 = Divide 0. 670 cm the cross section surface The fraction of the column that is swept by the mobile phase each minute is area covered by the mobile 1. 13 cm 3/min / 0. 670 cm 3 = 1. 69 min– 1 phase (π∙r 2∙ 0. 39) 1. 69 min– 1 · 10. 3 cm = 17. 4 cm/min

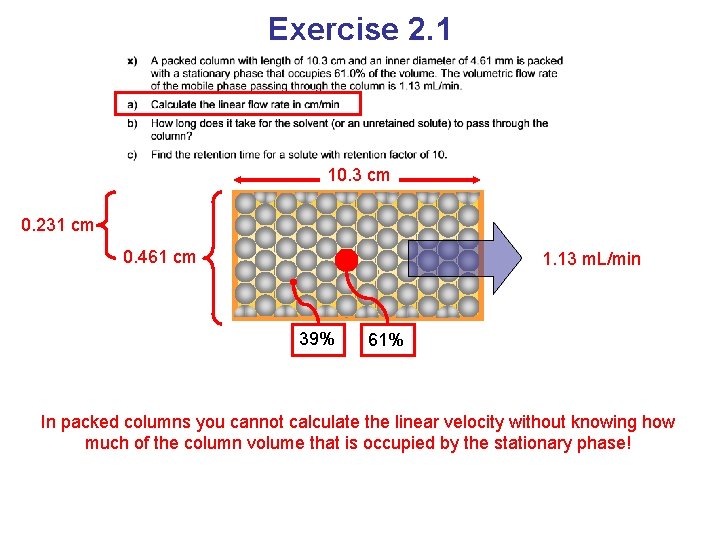
Exercise 2. 1 10. 3 cm 0. 231 cm 0. 461 cm 1. 13 m. L/min 39% 61% In packed columns you cannot calculate the linear velocity without knowing how much of the column volume that is occupied by the stationary phase!

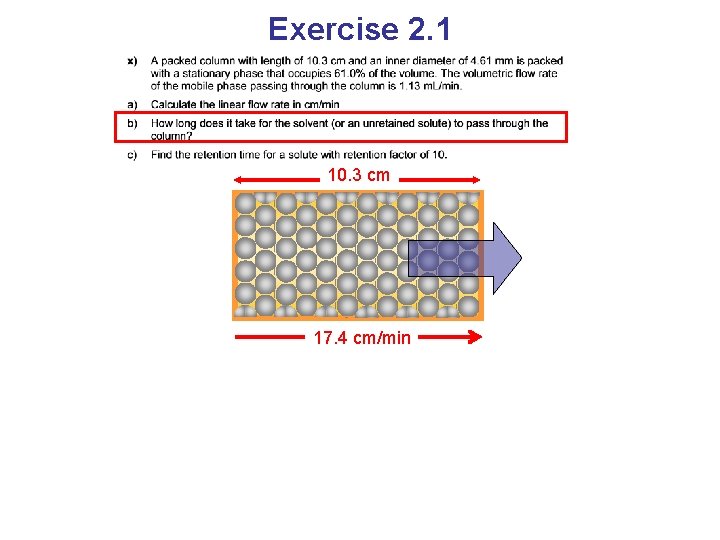
Exercise 2. 1 10. 3 cm 17. 4 cm/min

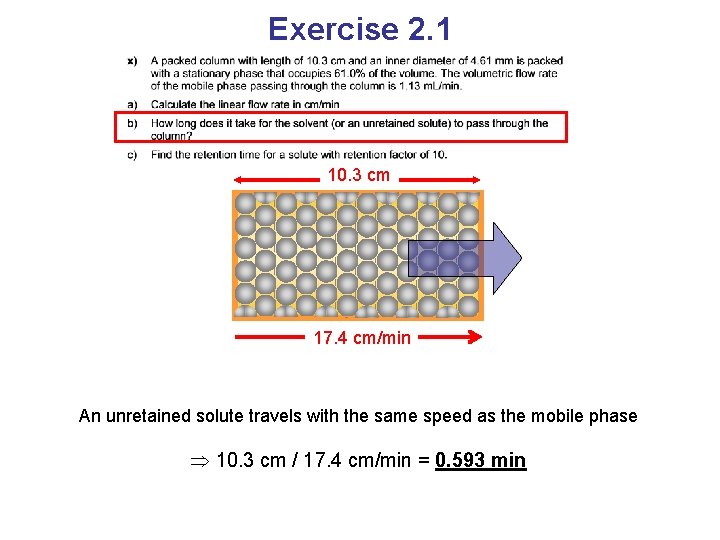
Exercise 2. 1 10. 3 cm 17. 4 cm/min An unretained solute travels with the same speed as the mobile phase 10. 3 cm / 17. 4 cm/min = 0. 593 min

Exercise 2. 1 10. 3 cm 17. 4 cm/min

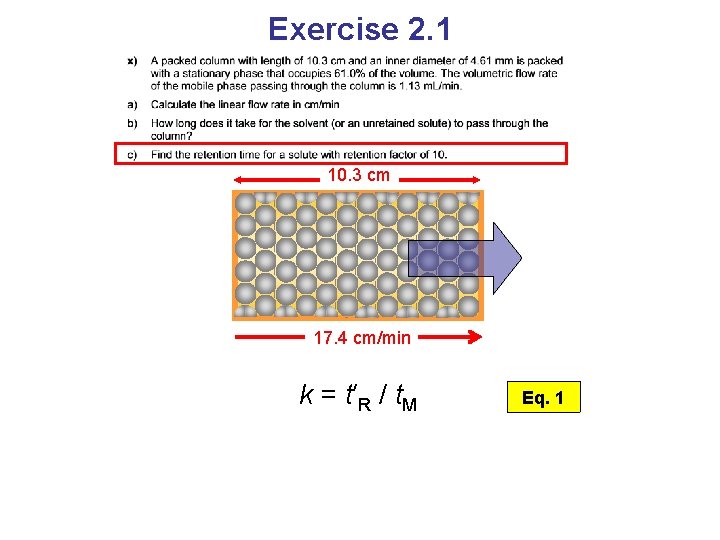
Exercise 2. 1 10. 3 cm 17. 4 cm/min k = t′R / t. M Eq. 1

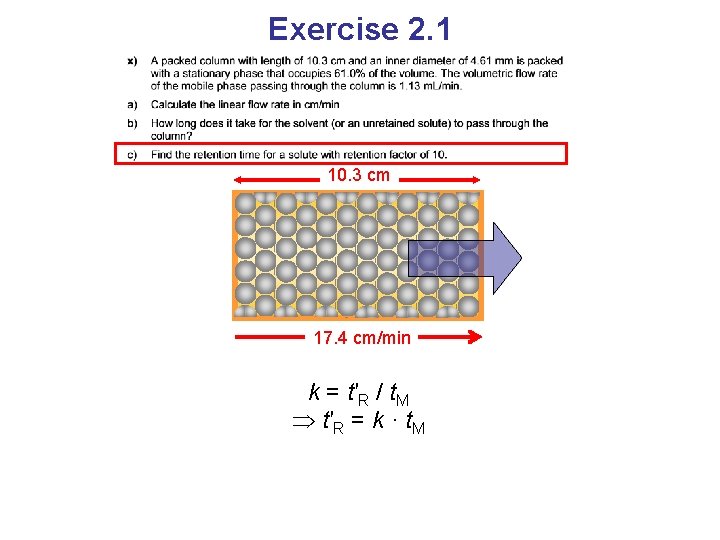
Exercise 2. 1 10. 3 cm 17. 4 cm/min k = t′R / t. M t′R = k · t. M

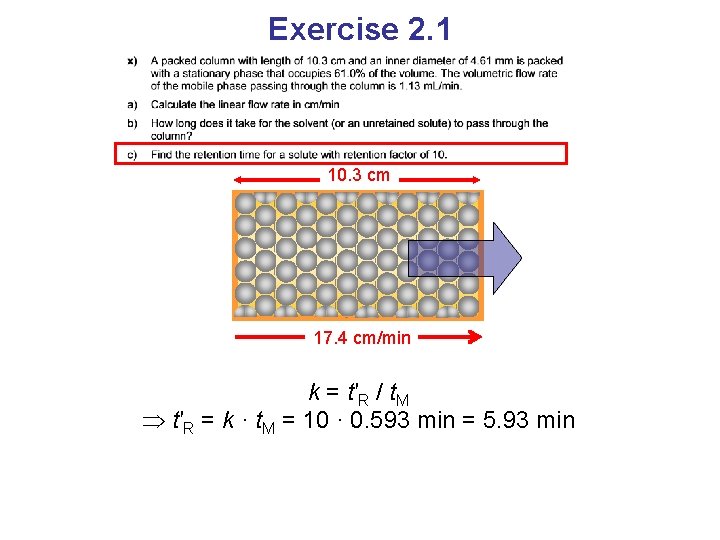
Exercise 2. 1 10. 3 cm 17. 4 cm/min k = t′R / t. M t′R = k · t. M = 10 · 0. 593 min = 5. 93 min

Exercise 2. 1 10. 3 cm 17. 4 cm/min k = t′R / t. M t′R = k · t. M = 10 · 0. 593 min = 5. 93 min t. R = t′R + t. M = 5. 93 min + 0. 593 min = 6. 53 min

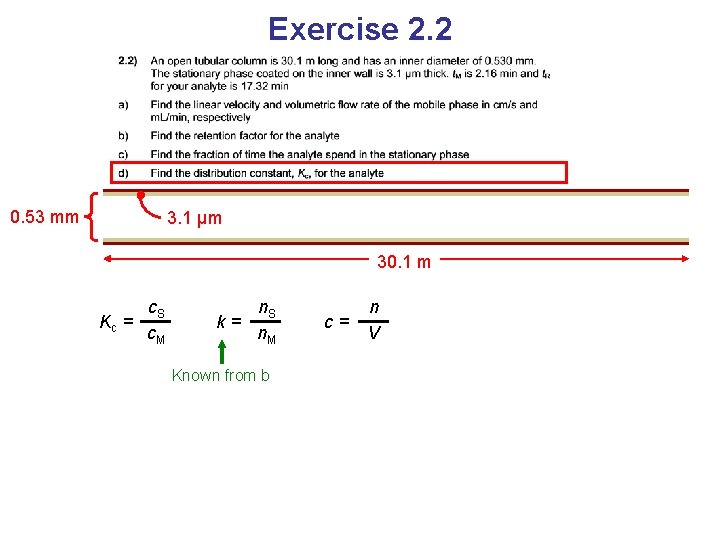
Exercise 2. 2

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m An unretained compound travels 30. 1 m in 2. 16 min u = 30. 1 m / 2. 16 min = 13. 9 m/min = 23. 2 cm/s

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m An unretained compound travels 30. 1 m in 2. 16 min u = 30. 1 m / 2. 16 min = 13. 9 m/min = 23. 2 cm/s The cross sectional area of the tube is πr 2 = 3. 14 · (0. 530/2)2 = 0. 221 mm 2 = 2. 21· 10– 3 cm 2

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m An unretained compound travels 30. 1 m in 2. 16 min u = 30. 1 m / 2. 16 min = 13. 9 m/min = 23. 2 cm/s The cross sectional area of the tube is πr 2 = 3. 14 · (0. 530/2)2 = 0. 221 mm 2 = 2. 21· 10– 3 cm 2 V / t = A · u = 2. 21· 10– 3 cm 2 · 1390 cm/min = 3. 07 cm 3/min

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m An unretained compound travels 30. 1 m in 2. 16 min u = 30. 1 m / 2. 16 min = 13. 9 m/min = 23. 2 cm/s The cross sectional area of the tube is πr 2 = 3. 14 · (0. 530/2)2 = 0. 221 mm 2 = 2. 21· 10– 3 cm 2 V / t = A · u = 2. 21· 10– 3 cm 2 · 1390 cm/min = 3. 07 cm 3/min

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m k= t. R′ t. M

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m k= t. R′ t. R – t. M = t. M

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m k= t. R′ t. R – t. M 17. 32 – 2. 16 = = = 7. 02 t. M 2. 16

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m k= t. R′ t. R – t. M 17. 32 – 2. 16 = = = 7. 02 t. M 2. 16 F= t. R′ t. R

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m k= t. R′ t. R – t. M 17. 32 – 2. 16 = = = 7. 02 t. M 2. 16 F= t. R′ t. R – t. M 17. 32 – 2. 16 = = = 0. 875 t. R 17. 32

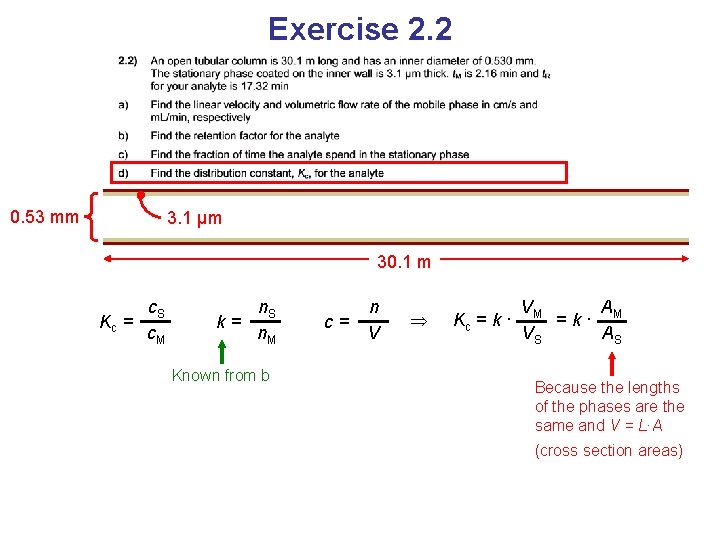
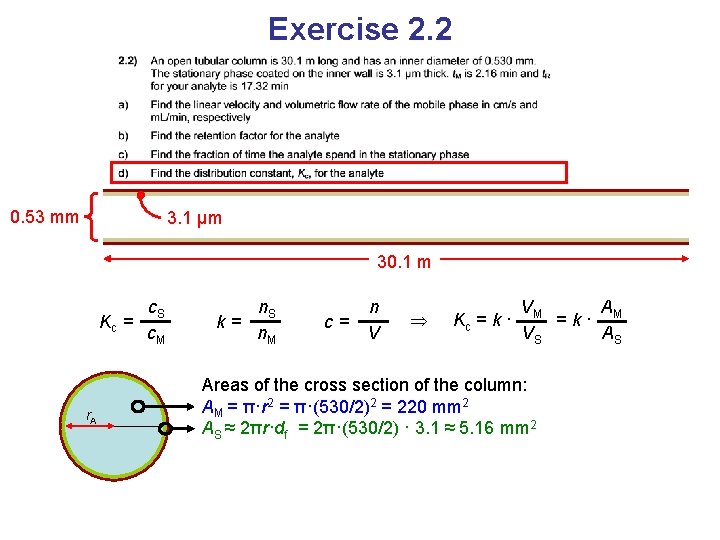
Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Kc = c. S c. M Distribution constant

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Kc = c. S c. M k= n. S n. M Known from b c= n V

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Kc = c. S c. M k= n. S n. M Known from b c= n V Kc = k · VM A =k· M VS AS Because the lengths of the phases are the same and V = L∙A (cross section areas)

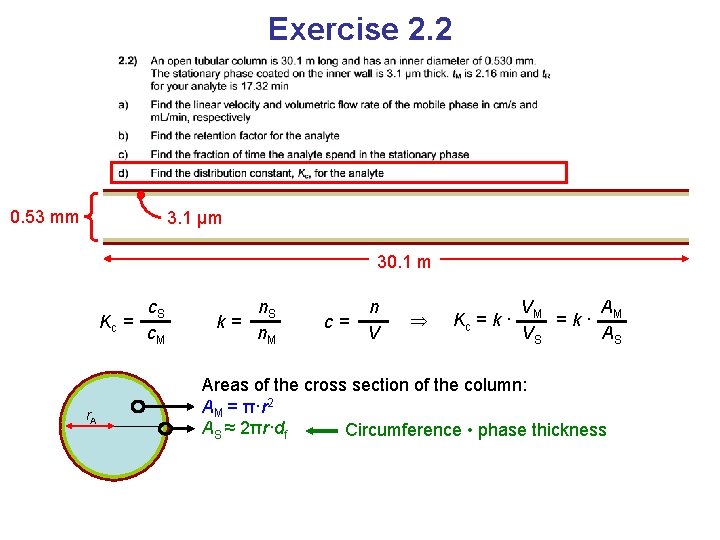
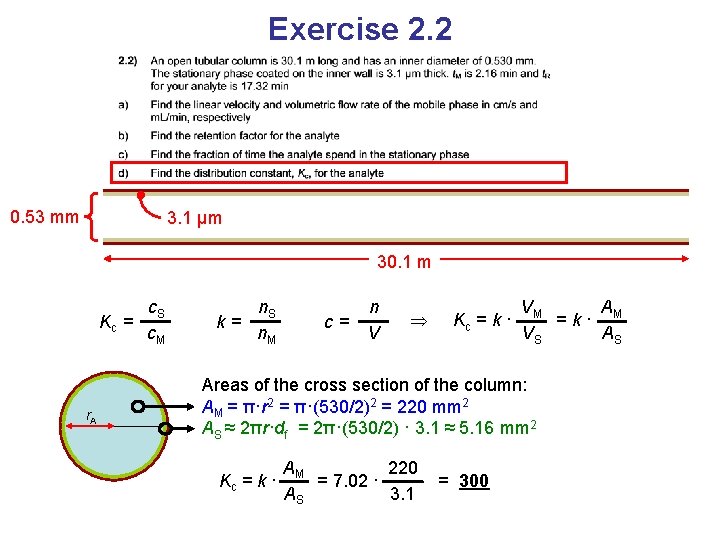
Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Kc = r. A c. S c. M k= n. S n. M c= n V Kc = k · VM A =k· M VS AS Areas of the cross section of the column: AM = π·r 2 AS ≈ 2πr·df Circumference • phase thickness

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Kc = r. A c. S c. M k= n. S n. M c= n V Kc = k · VM A =k· M VS AS Areas of the cross section of the column: AM = π·r 2 = π·(530/2)2 = 220 mm 2 AS ≈ 2πr·df = 2π·(530/2) · 3. 1 ≈ 5. 16 mm 2

Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Kc = r. A c. S c. M k= n. S n. M c= n V Kc = k · VM A =k· M VS AS Areas of the cross section of the column: AM = π·r 2 = π·(530/2)2 = 220 mm 2 AS ≈ 2πr·df = 2π·(530/2) · 3. 1 ≈ 5. 16 mm 2 Kc = k · AM 220 = 7. 02 · AS 3. 1 = 300

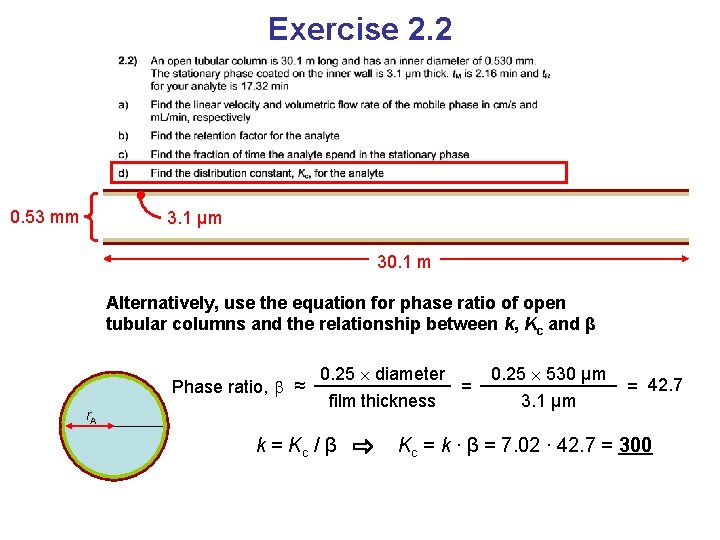
Exercise 2. 2 0. 53 mm 3. 1 μm 30. 1 m Alternatively, use the equation for phase ratio of open tubular columns and the relationship between k, Kc and β Phase ratio, ≈ r. A 0. 25 diameter film thickness k = Kc / β = 0. 25 530 µm 3. 1 µm = 42. 7 Kc = k ∙ β = 7. 02 ∙ 42. 7 = 300

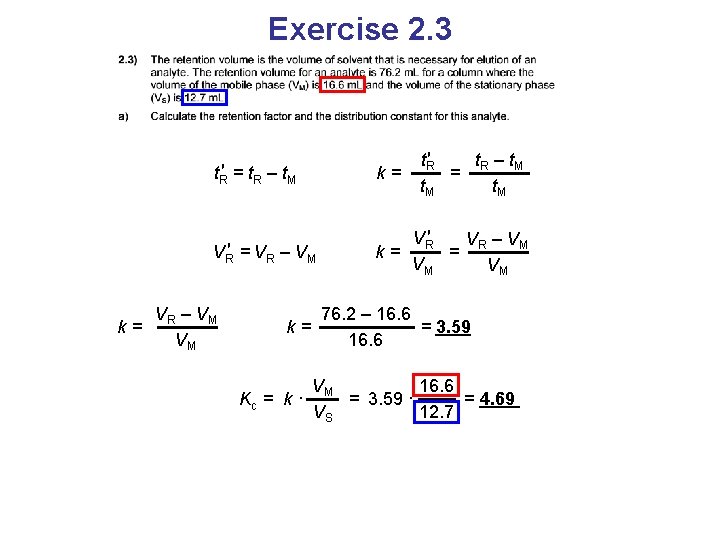
Exercise 2. 3

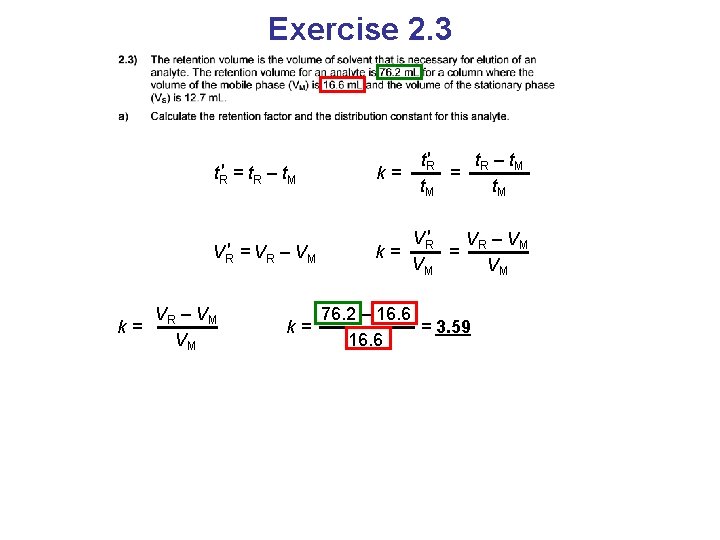
Exercise 2. 3 t. R′ = t. R – t. M k= t. R′ t –t = R M t. M

Exercise 2. 3 t. R′ t –t = R M t. R′ = t. R – t. M k= VR′ = VR – VM VR′ V – VM k= = R VM VM

Exercise 2. 3 t. R′ t –t = R M t. R′ = t. R – t. M k= VR′ = VR – VM VR′ V – VM k= = R VM VM VR – VM k= VM 76. 2 – 16. 6 k= = 3. 59 16. 6

Exercise 2. 3 t. R′ t –t = R M t. R′ = t. R – t. M k= VR′ = VR – VM VR′ V – VM k= = R VM VM VR – VM k= VM 76. 2 – 16. 6 k= = 3. 59 16. 6 VM 16. 6 Kc = k · = 3. 59 · = 4. 69 VS 12. 7

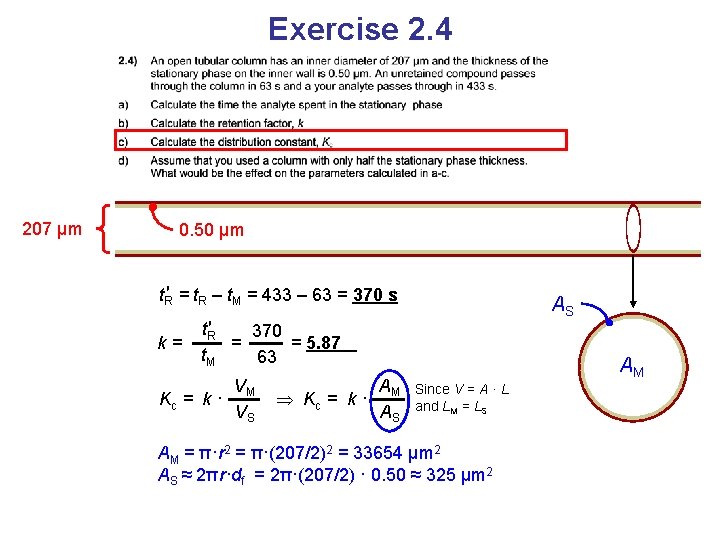
Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= AS t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS AM Kc = k · AS AM Since V = A · L and LM = LS AM = π·r 2 = π·(207/2)2 = 33654 μm 2 AS ≈ 2πr·df = 2π·(207/2) · 0. 50 ≈ 325 μm 2

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS AS AM 33654 Kc = k · =k· = 608 AS 325 AM = π·r 2 = π·(207/2)2 = 33654 μm 2 AS ≈ 2πr·df = 2π·(207/2) · 0. 50 ≈ 325 μm 2 AM

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 AS AM VM Kc = k · VS AM 33654 Kc = k · =k· = 608 AS 325 AM = π·r 2 AS ≈ 2πr·df If we decrease df to half, we decrease AS and VS to half Other dimensions remain the same

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS AS k = Kc · AM The distribution constant, Kc is independent of the column dimensions and therefore remains the same AS AM

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS AS k = Kc · AM Since AS is reduced to half, k is reduced to half since both K and AM is unaffected new k = 2. 94 AS AM

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS AS k = Kc · AM t. M is unaffected by the phase thickness and t′R must be reduced to half for k to be halved New t′R is 185 s AS AM

Exercise 2. 4 207 μm 0. 50 μm t. R′ = t. R – t. M = 433 – 63 = 370 s k= t. R′ 370 = = 5. 87 t. M 63 VM Kc = k · VS AS k = Kc · AM t. M is unaffected by the phase thickness and t′R must be reduced to half for k to be halved New t′R is 185 s (and new t. R is 185 s + 63 s = 248 s) AS AM

Exercise 2. 5 k = Kc · VS VM

Exercise 2. 5 k = Kc · VS VM Increasing the volume of stationary phase relative to the mobile phase will increase k

Exercise 2. 5 k = Kc · VS VM Increasing the volume of stationary phase relative to the mobile phase will increase k Increasing the volume of mobile phase relative to stationary phase will decrease k

Exercise 2. 5 k = Kc · VS VM Increasing the volume of stationary phase relative to the mobile phase will increase k Increasing the volume of mobile phase relative to stationary phase will decrease k Higher solvent strength in LC will decrease Kc and therefore also k Higher temperature in GC will decrease Kc and therefore also k

Exercise 2. 5 k = Kc · VS VM Increasing the volume of stationary phase relative to the mobile phase will increase k Increasing the volume of mobile phase relative to stationary phase will decrease k Higher solvent strength in LC will decrease Kc and therefore also k Higher temperature in GC will decrease Kc and therefore also k
