Exercise 9 Typical Gas Problems where n is
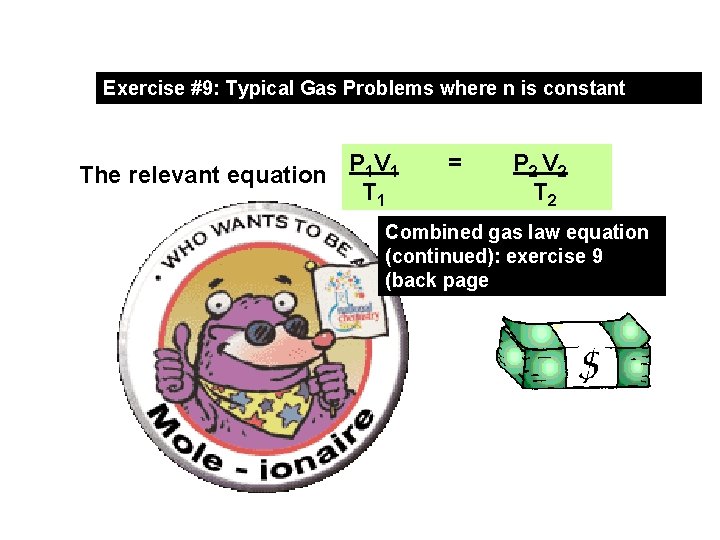
Exercise #9: Typical Gas Problems where n is constant The relevant equation P 1 V 1 T 1 = P 2 V 2 T 2 Combined gas law equation (continued): exercise 9 (back page
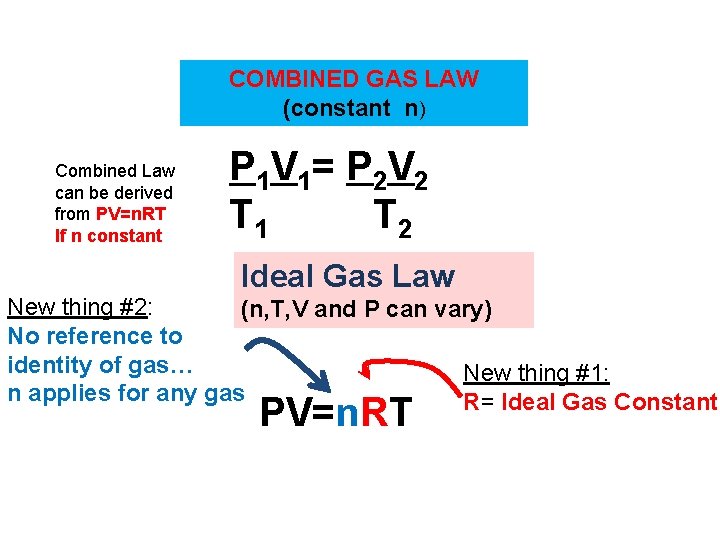
COMBINED GAS LAW (constant n) Combined Law can be derived from PV=n. RT If n constant P 1 V 1= P 2 V 2 T 1 T 2 Ideal Gas Law New thing #2: (n, T, V and P can vary) No reference to identity of gas… New thing #1: n applies for any gas R= Ideal Gas Constant PV=n. RT

All 4 gas variables are explicit R= 0. 08206 atm L/K mole Chemists’ prefer this unit Cats rule, dogs drool ? ? R= 8. 314 J/K mole physicists’ prefer this unit
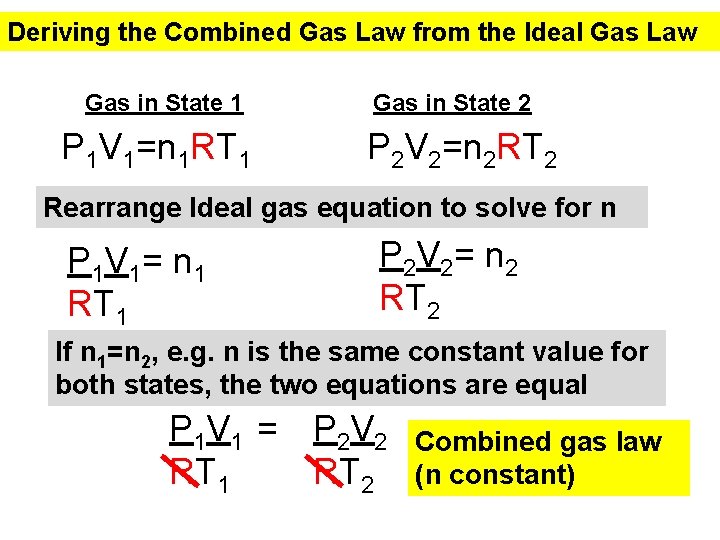
Deriving the Combined Gas Law from the Ideal Gas Law Gas in State 1 P 1 V 1=n 1 RT 1 Gas in State 2 P 2 V 2=n 2 RT 2 Rearrange Ideal gas equation to solve for n P 1 V 1= n 1 RT 1 P 2 V 2= n 2 RT 2 If n 1=n 2, e. g. n is the same constant value for both states, the two equations are equal P 1 V 1 = P 2 V 2 RT 1 RT 2 Combined gas law (n constant)
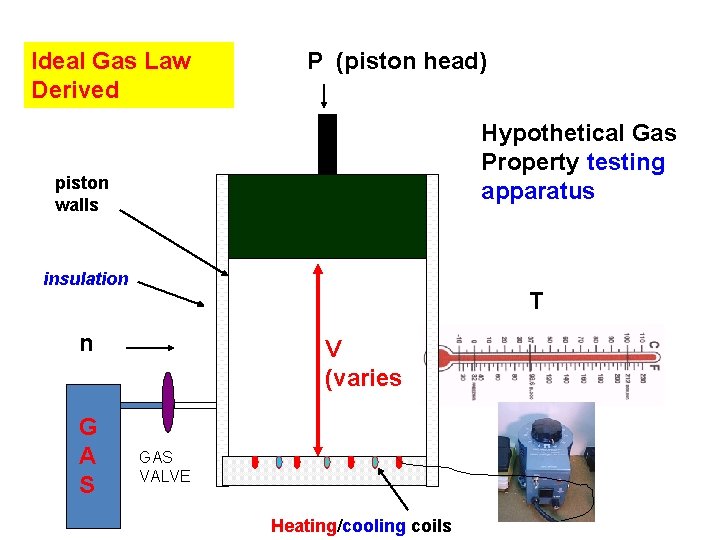
Ideal Gas Law Derived P (piston head) Hypothetical Gas Property testing apparatus piston walls insulation T n G A S V (varies GAS VALVE Heating/cooling coils
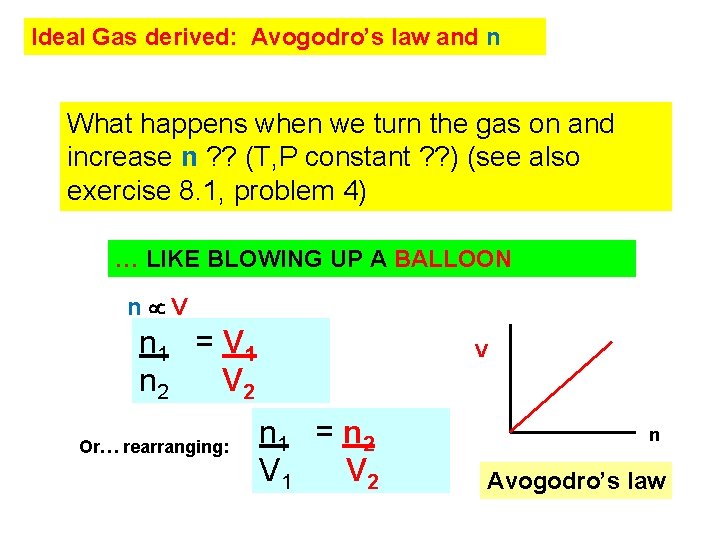
Ideal Gas derived: Avogodro’s law and n What happens when we turn the gas on and increase n ? ? (T, P constant ? ? ) (see also exercise 8. 1, problem 4) … LIKE BLOWING UP A BALLOON n V n 1 = V 1 n 2 V 2 Or… rearranging: V n 1 = n 2 V 1 V 2 n Avogodro’s law
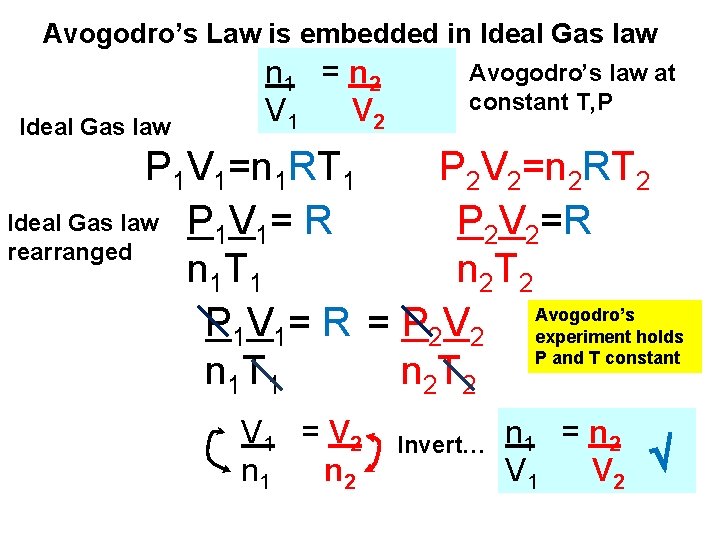
Avogodro’s Law is embedded in Ideal Gas law n 1 = n 2 V 1 V 2 Avogodro’s law at constant T, P P 1 V 1=n 1 RT 1 P 2 V 2=n 2 RT 2 Ideal Gas law P V = R P 2 V 2=R 1 1 rearranged n 1 T 1 n 2 T 2 Avogodro’s P 1 V 1= R = P 2 V 2 experiment holds P and T constant n 1 T 1 n 2 T 2 V 1 = V 2 n 1 n 2 Invert… n 1 = n 2 V 1 V 2
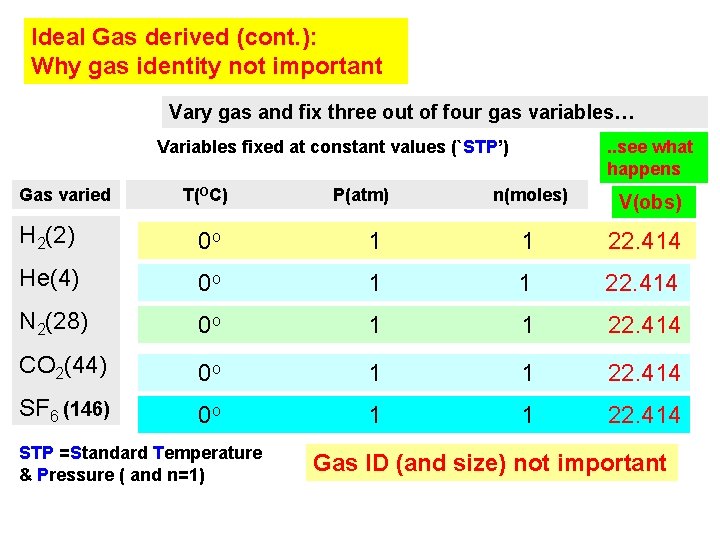
Ideal Gas derived (cont. ): Why gas identity not important Vary gas and fix three out of four gas variables… Variables fixed at constant values (`STP’) Gas varied T(OC) P(atm) . . see what happens n(moles) V(obs) H 2(2) 0 o 1 1 22. 414 He(4) 0 o 1 1 22. 414 N 2(28) 0 o 1 1 22. 414 CO 2(44) 0 o 1 1 22. 414 SF 6 (146) 0 o 1 1 22. 414 STP =Standard Temperature & Pressure ( and n=1) Gas ID (and size) not important
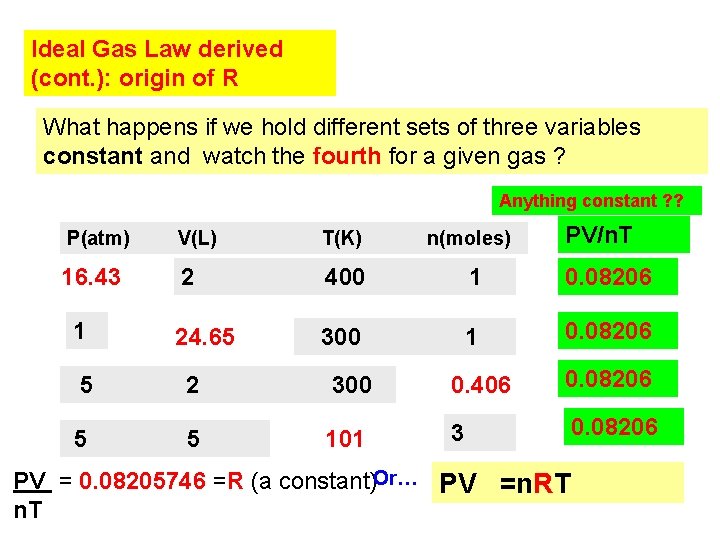
Ideal Gas Law derived (cont. ): origin of R What happens if we hold different sets of three variables constant and watch the fourth for a given gas ? Anything constant ? ? V(L) T(K) 16. 43 2 400 1 0. 08206 24. 65 300 1 0. 08206 1 n(moles) PV/n. T P(atm) 5 2 300 0. 406 0. 08206 5 5 101 3 0. 08206 PV = 0. 08205746 =R (a constant)Or… PV =n. RT n. T
Exercise #9: Ideal gas law problems…when n is the focus Exercise 9. 1 problem 4 Exercise 9. 3 problems 1 3 The relevant equation: PV=n. RT
- Slides: 10