Examples of systematic reviews Goran Poropat Cochrane systematic













- Slides: 32

Examples of systematic reviews Goran Poropat

Cochrane systematic reviews To make unmanageable amounts of information – manageable Identify, appraise and synthesize research-based evidence Present in an accessible format

Cochrane systematic reviews Clearly stated set of objectives Explicit, reproducible methodology Systematic search Assessment of validity Systematic presentation


Protocol for a Cochrane review Idea Register title Write protocol To minimize the potential for bias in the review process Changes possible

Bezafibrate for primary biliary cirrhosis Title* Protocol information: Authors* Contact person* Dates What’s new History The protocol: Background* Objectives* Methods: Criteria for selecting studies for this review: Types of studies* Types of participants* Types of interventions* Types of outcome measures* Search methods for identification of studies* Data collection and analysis* Acknowledgements References: Other references: Additional references Other published versions of this review Tables and figures: Additional tables Figures Supplementary information: Appendices Feedback: Title Summary Reply Contributors About the article: Contributions of authors Declarations of interest* Sources of support: Internal sources External sources Published notes

Bezafibrate for primary biliary cirrhosis

Bezafibrate for primary biliary cirrhosis • Chronic, progressive, inflammatory and autoimmunemediated liver disease • Survival in symptomatic patients = 10 -15 yr. • Bilirubin – indipendent predictor of survival and prognosis • Bezafibrate – inhibition of acetil-Co. A carboxylase activity • PPAR - ATP-binding cassette (ABC) B 4 transporter • Increased secretion of phosphatidyl-choline

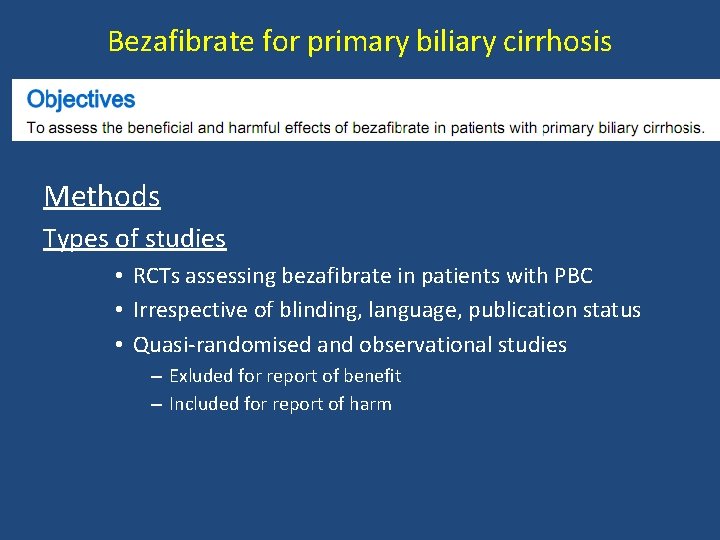
Bezafibrate for primary biliary cirrhosis Methods Types of studies • RCTs assessing bezafibrate in patients with PBC • Irrespective of blinding, language, publication status • Quasi-randomised and observational studies – Exluded for report of benefit – Included for report of harm

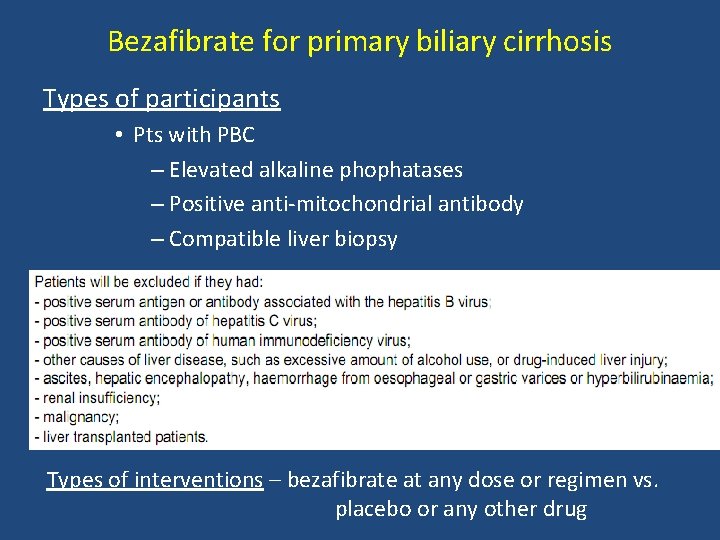
Bezafibrate for primary biliary cirrhosis Types of participants • Pts with PBC – Elevated alkaline phophatases – Positive anti-mitochondrial antibody – Compatible liver biopsy Types of interventions – bezafibrate at any dose or regimen vs. placebo or any other drug

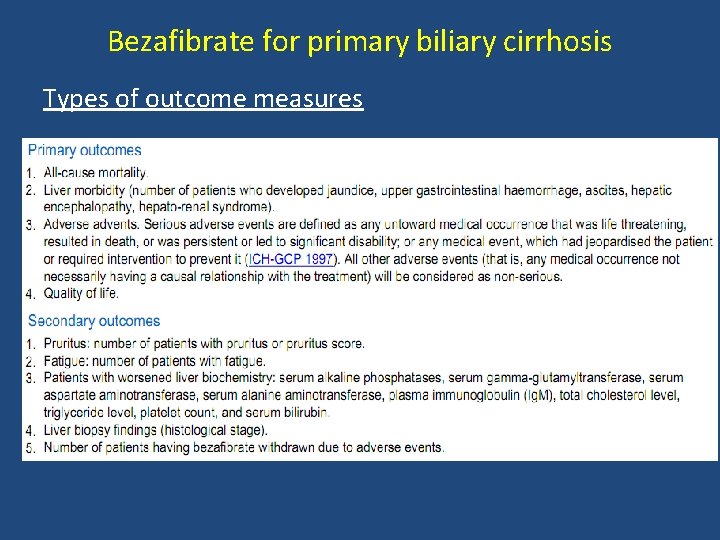
Bezafibrate for primary biliary cirrhosis Types of outcome measures

Bezafibrate for primary biliary cirrhosis Search methods of identification of studies • Cochrane Hepato-Biliary Group Controlled Trials Register • The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library • MEDLINE • EMBASE • Science Citation Index Expanded • LILACS • Chinese Bio-medical Literature Database • • Reference lists of identified studies Pharmaceutical companies Clinicaltrials. gov WHO International Clinical Trials Registry Platform

Bezafibrate for primary biliary cirrhosis Data collection and analysis • Two authors independently • Disagreements resolved by discussion Risk of bias assessment • • • Allocation sequence generation Allocation concealment Blinding Incomplete outcome data Selective outcome reporting Other bias

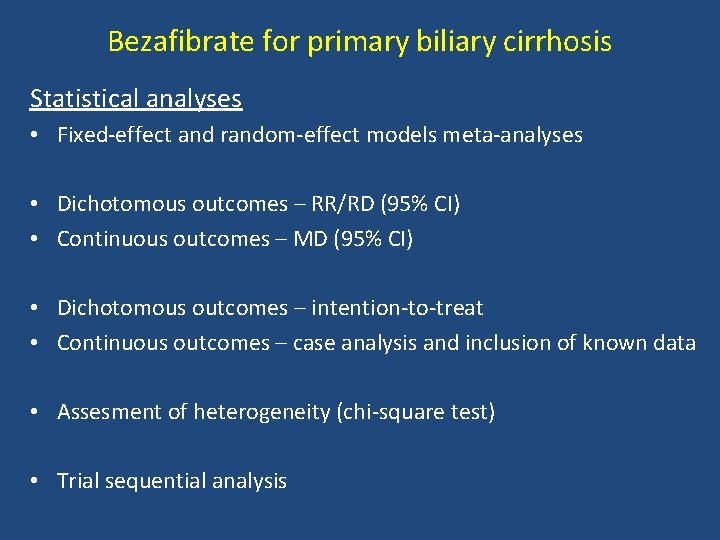
Bezafibrate for primary biliary cirrhosis Statistical analyses • Fixed-effect and random-effect models meta-analyses • Dichotomous outcomes – RR/RD (95% CI) • Continuous outcomes – MD (95% CI) • Dichotomous outcomes – intention-to-treat • Continuous outcomes – case analysis and inclusion of known data • Assesment of heterogeneity (chi-square test) • Trial sequential analysis

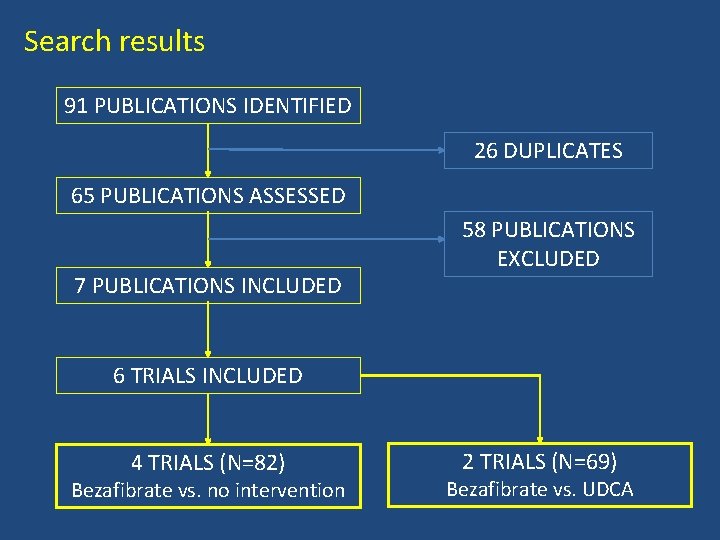
Search results 91 PUBLICATIONS IDENTIFIED 26 DUPLICATES 65 PUBLICATIONS ASSESSED 7 PUBLICATIONS INCLUDED 58 PUBLICATIONS EXCLUDED 6 TRIALS INCLUDED 4 TRIALS (N=82) Bezafibrate vs. no intervention 2 TRIALS (N=69) Bezafibrate vs. UDCA

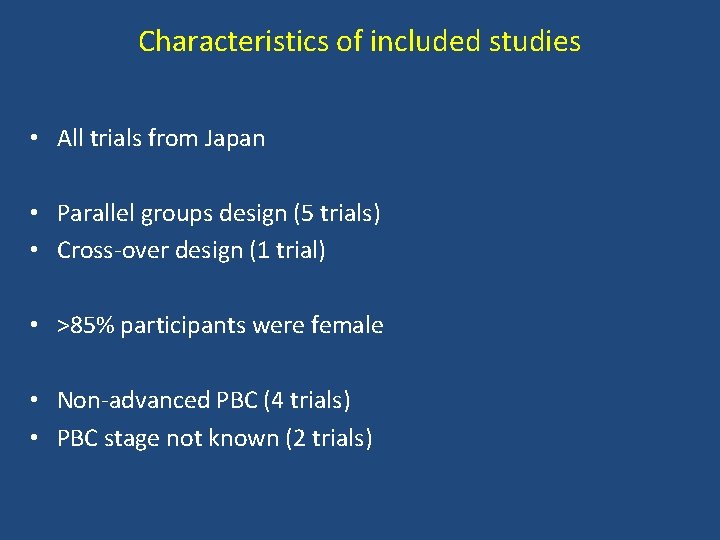
Characteristics of included studies • All trials from Japan • Parallel groups design (5 trials) • Cross-over design (1 trial) • >85% participants were female • Non-advanced PBC (4 trials) • PBC stage not known (2 trials)

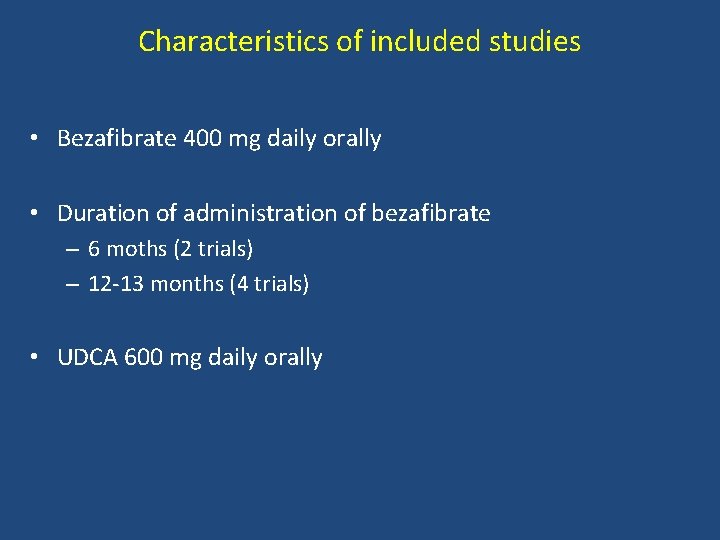
Characteristics of included studies • Bezafibrate 400 mg daily orally • Duration of administration of bezafibrate – 6 moths (2 trials) – 12 -13 months (4 trials) • UDCA 600 mg daily orally

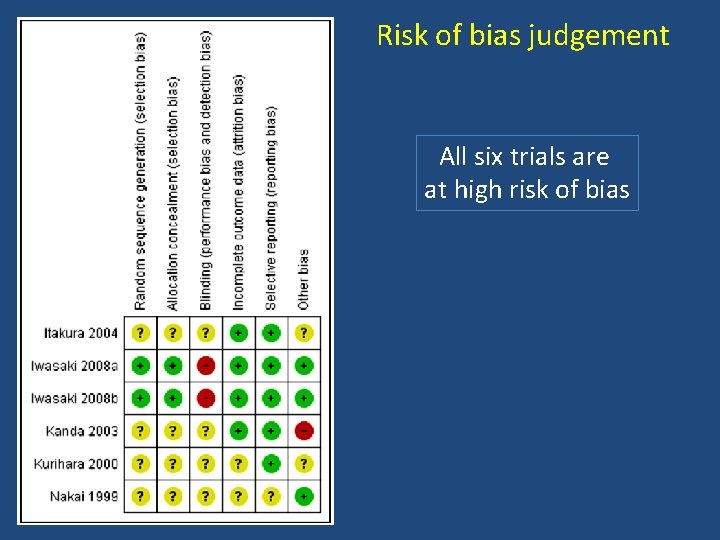
Risk of bias judgement All six trials are at high risk of bias

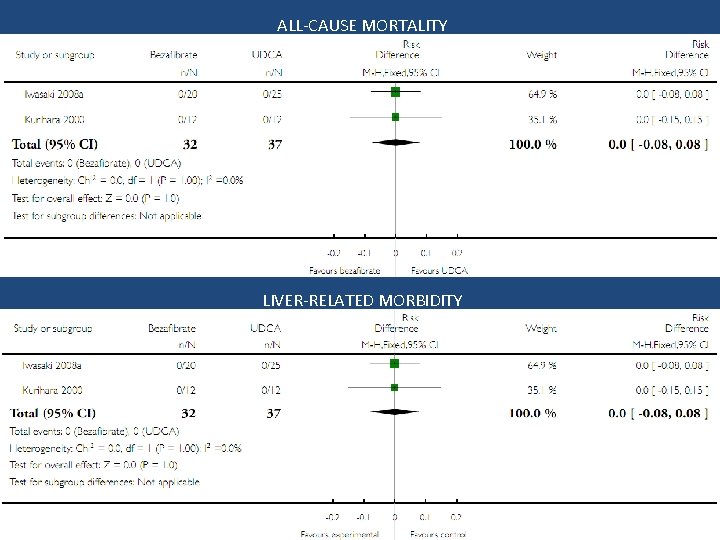
Results: Trials assessing bezafibrate vs. no intervention

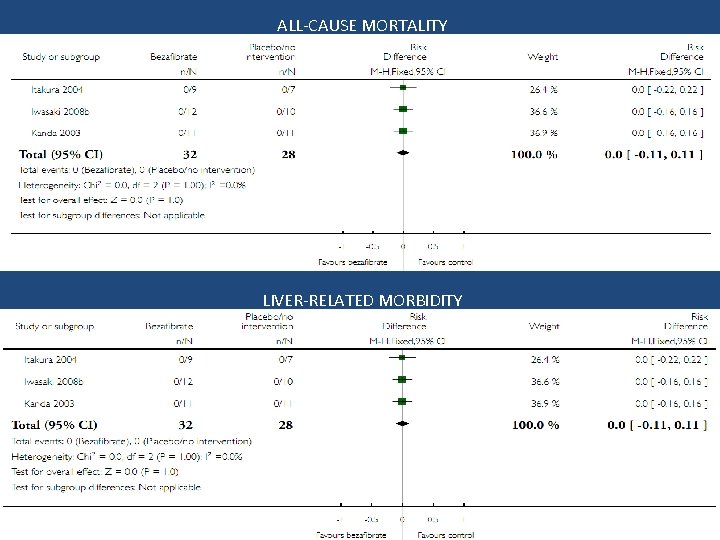
ALL-CAUSE MORTALITY LIVER-RELATED MORBIDITY

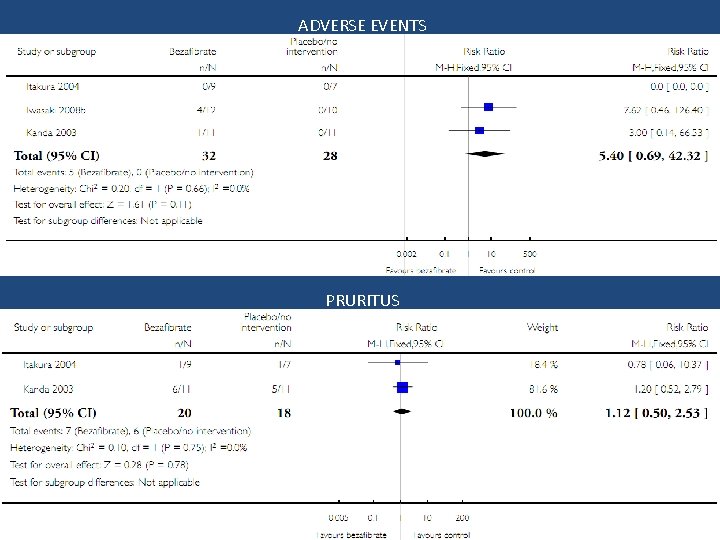
ADVERSE EVENTS PRURITUS

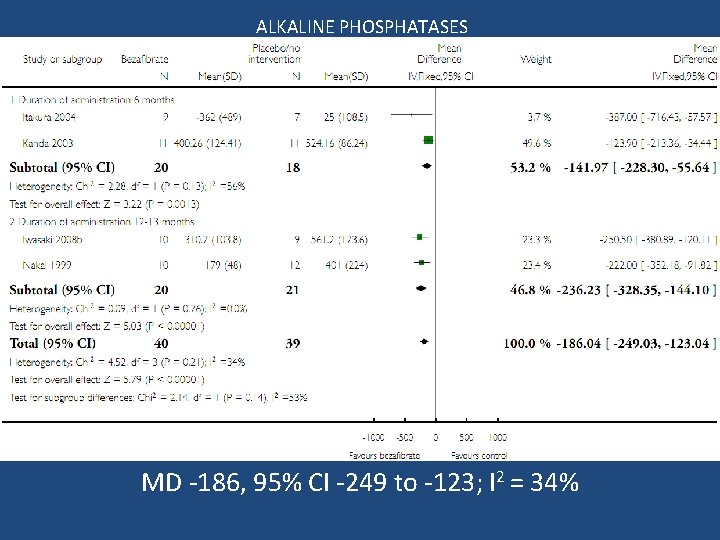
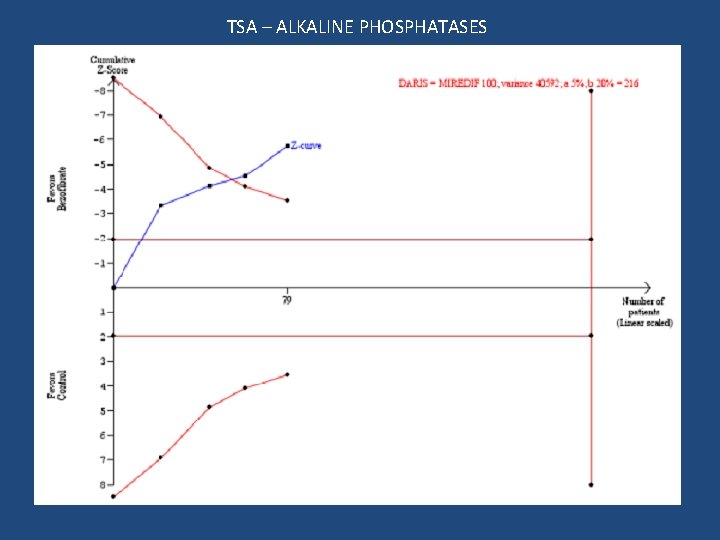
ALKALINE PHOSPHATASES MD -186, 95% CI -249 to -123; I 2 = 34%

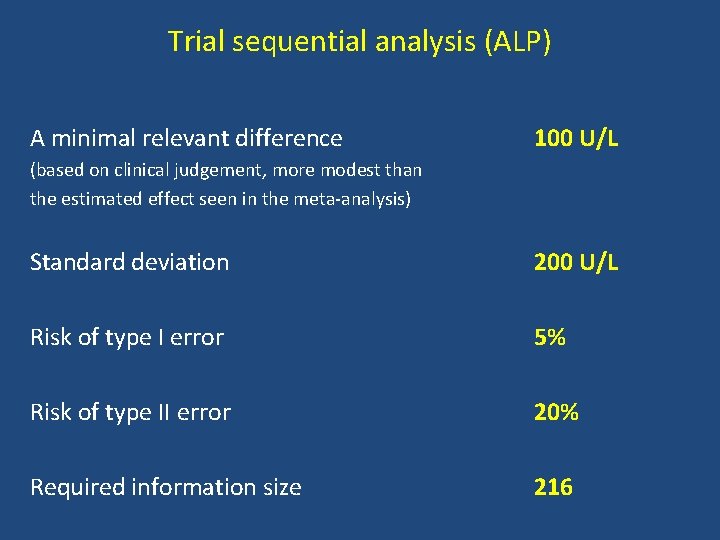
Trial sequential analysis (ALP) A minimal relevant difference 100 U/L (based on clinical judgement, more modest than the estimated effect seen in the meta-analysis) Standard deviation 200 U/L Risk of type I error 5% Risk of type II error 20% Required information size 216

TSA – ALKALINE PHOSPHATASES

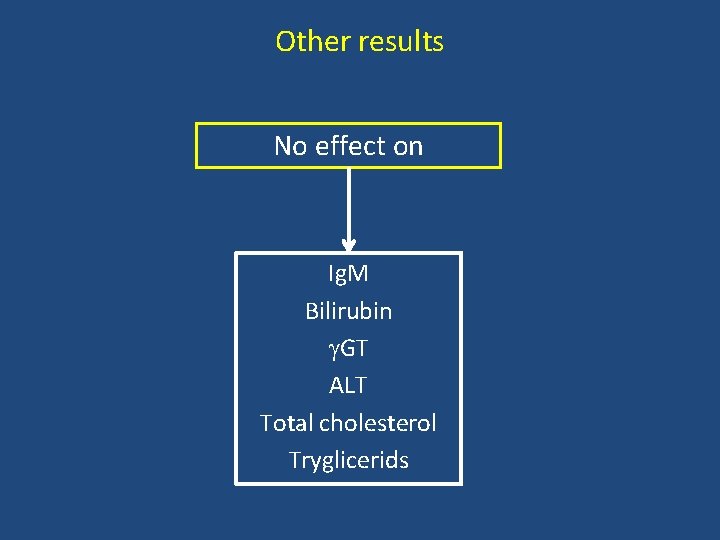
Other results No effect on Ig. M Bilirubin GT ALT Total cholesterol Tryglicerids

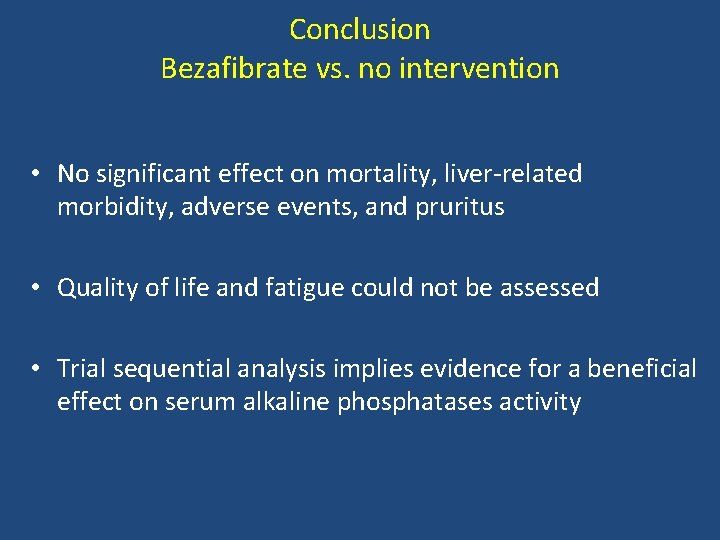
Conclusion Bezafibrate vs. no intervention • No significant effect on mortality, liver-related morbidity, adverse events, and pruritus • Quality of life and fatigue could not be assessed • Trial sequential analysis implies evidence for a beneficial effect on serum alkaline phosphatases activity

Results: Trials assessing bezafibrate vs. ursodeoxycholic acid (UDCA)

ALL-CAUSE MORTALITY LIVER-RELATED MORBIDITY

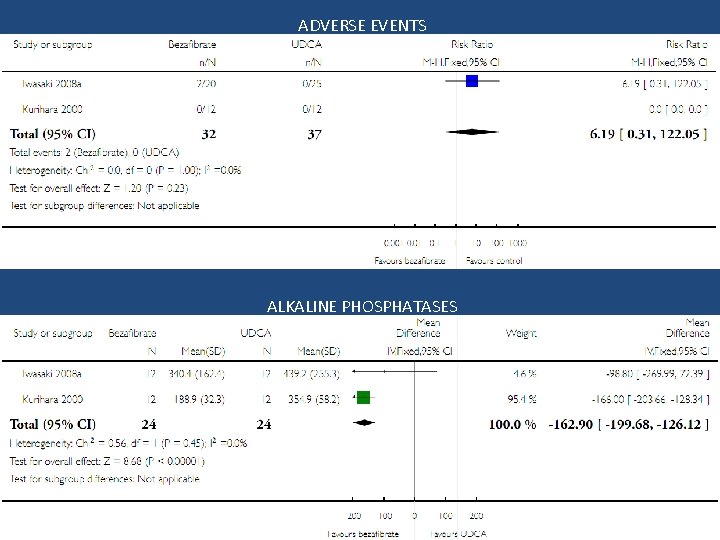
ADVERSE EVENTS ALKALINE PHOSPHATASES

Other results Fixed-effect analysis Random-effect analysis Significant decrease of ALP, GT, ALT, Ig. M No effect on GT, ALT, Ig. M

Conclusion Bezafibrate vs. UDCA • No significant effect on mortality, liver-related morbidity, adverse events, and pruritus • Quality of life, pruritus, and fatigue could not be assessed • There is no firm evidence of effect on ALP, GT, ALT, and Ig. M

CONCLUSIONS Treatment of primary biliary cirrhosis with bezafibrate can neither be supported nor rejected
 Gt performance goran
Gt performance goran Prospero systematic review
Prospero systematic review 5 stappen ebp nursing
5 stappen ebp nursing Mark wilson cochrane
Mark wilson cochrane Cochrane risk of bias table template
Cochrane risk of bias table template Metanalise o que é
Metanalise o que é Cochrane metaanalyse
Cochrane metaanalyse Cochrane training
Cochrane training Marianne cochrane
Marianne cochrane Euan cochrane
Euan cochrane Mecir cochrane
Mecir cochrane Vavasseur
Vavasseur Riesgo de sesgo
Riesgo de sesgo Sesgo de notificacion
Sesgo de notificacion Cochrane lions football
Cochrane lions football Cochrane wiley
Cochrane wiley Cochrane
Cochrane 027731
027731 Manual cochrane
Manual cochrane Cochrane rapid review
Cochrane rapid review Cochrane musculoskeletal group
Cochrane musculoskeletal group Goran repinc
Goran repinc Vinograd pjesma
Vinograd pjesma Goran milas
Goran milas Goran vesi
Goran vesi Hipertenzija kod mladih
Hipertenzija kod mladih Bankartova lezija
Bankartova lezija Goran pavlov
Goran pavlov Tihana galinac grbac
Tihana galinac grbac Goran nenadic
Goran nenadic Mali pot ivan goran kovačić analiza pjesme
Mali pot ivan goran kovačić analiza pjesme Goran sirovatka
Goran sirovatka Nodalni ritam
Nodalni ritam























































