Example of Health Technology Assessment HTA of a









- Slides: 18

Example of Health Technology Assessment (HTA) of a therapy for the reduction of alcohol consumption David Tyas Global HEOR - Lundbeck

Contents • Introduction into a HTA process • • Use SMC as an example but equally could be from many other countries Summary of our submission Main argument • Types of analysis • Clarification question stage • • • Summary of questions (what sort) Final recommendation 2

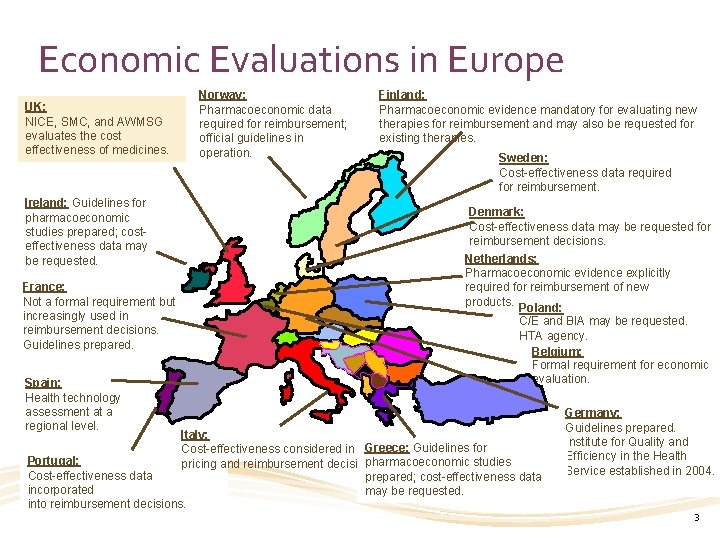
Economic Evaluations in Europe UK: NICE, SMC, and AWMSG evaluates the cost effectiveness of medicines. Ireland: Guidelines for pharmacoeconomic studies prepared; costeffectiveness data may be requested. France: Not a formal requirement but increasingly used in reimbursement decisions. Guidelines prepared. Spain: Health technology assessment at a regional level. Norway: Pharmacoeconomic data required for reimbursement; official guidelines in operation. Finland: Pharmacoeconomic evidence mandatory for evaluating new therapies for reimbursement and may also be requested for existing therapies. Sweden: Cost-effectiveness data required for reimbursement. Denmark: Cost-effectiveness data may be requested for reimbursement decisions. Netherlands: Pharmacoeconomic evidence explicitly required for reimbursement of new products. Poland: C/E and BIA may be requested. HTA agency. Belgium: Formal requirement for economic evaluation. Italy: Cost-effectiveness considered in Greece: Guidelines for Portugal: pharmacoeconomic studies pricing and reimbursement decisions. Cost-effectiveness data prepared; cost-effectiveness data incorporated may be requested. into reimbursement decisions. Germany: Guidelines prepared. Institute for Quality and Efficiency in the Health Service established in 2004. 3

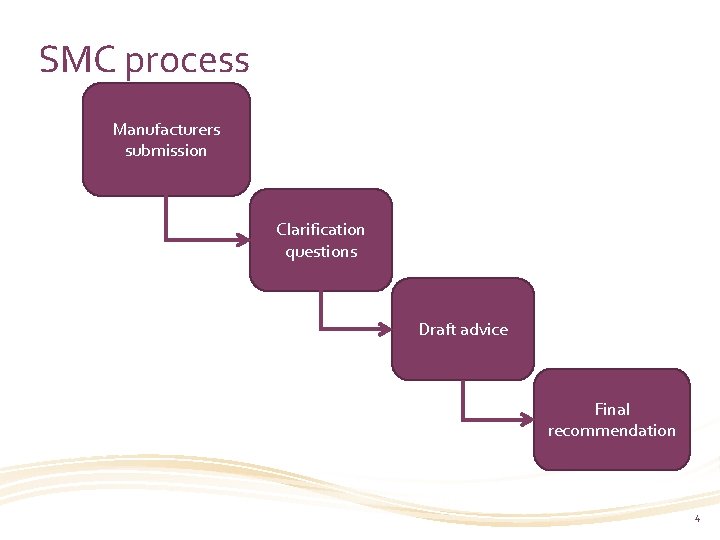
SMC process Manufacturers submission Clarification questions Draft advice Final recommendation 4

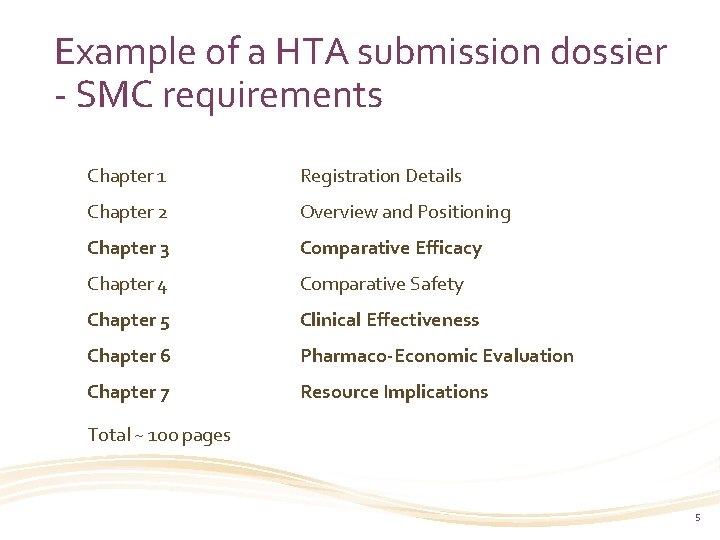
Example of a HTA submission dossier - SMC requirements Chapter 1 Registration Details Chapter 2 Overview and Positioning Chapter 3 Comparative Efficacy Chapter 4 Comparative Safety Chapter 5 Clinical Effectiveness Chapter 6 Pharmaco-Economic Evaluation Chapter 7 Resource Implications Total ~ 100 pages 5

Nalmefene Main arguments and data 6

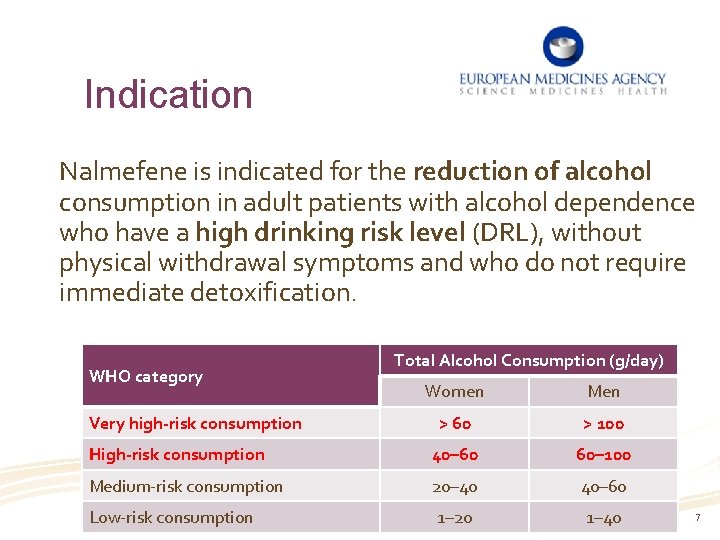
Indication Nalmefene is indicated for the reduction of alcohol consumption in adult patients with alcohol dependence who have a high drinking risk level (DRL), without physical withdrawal symptoms and who do not require immediate detoxification. WHO category Total Alcohol Consumption (g/day) Women Men > 60 > 100 High-risk consumption 40– 60 60– 100 Medium-risk consumption 20– 40 40– 60 Low-risk consumption 1– 20 1– 40 Very high-risk consumption 7

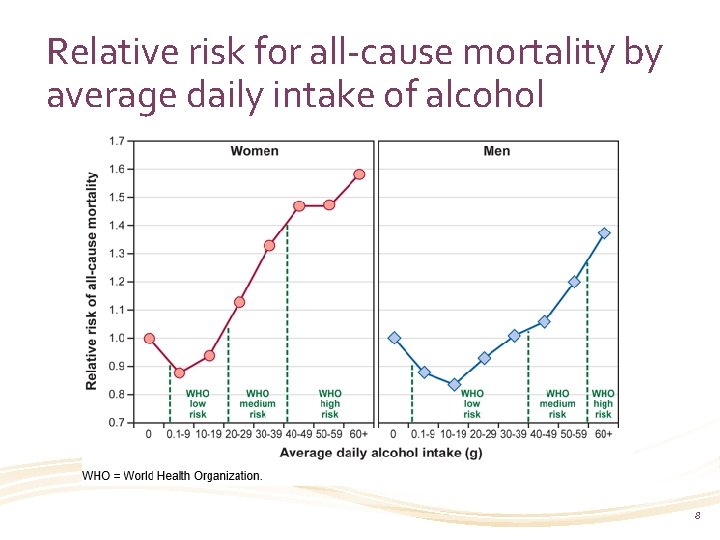
Relative risk for all-cause mortality by average daily intake of alcohol 8

9

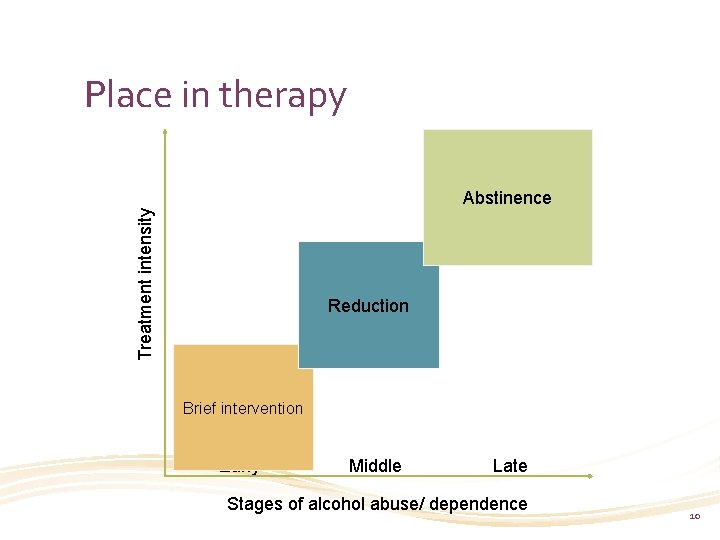
Place in therapy Treatment intensity Abstinence Reduction Brief intervention Early Middle Late Stages of alcohol abuse/ dependence 10

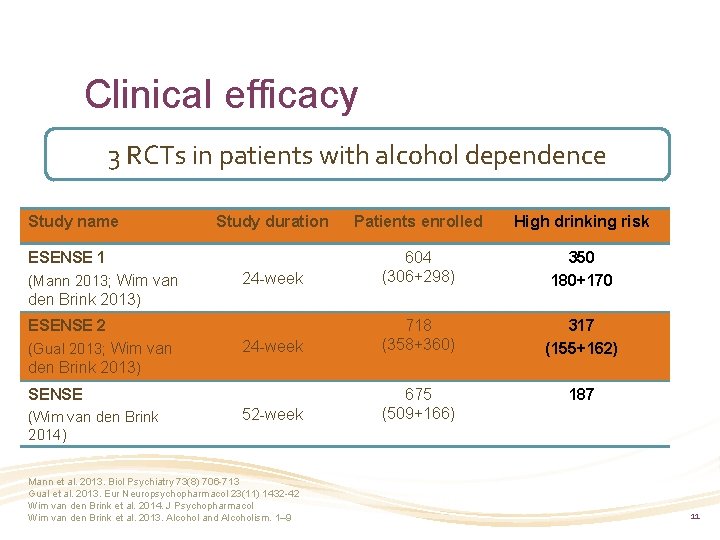
Clinical efficacy 3 RCTs in patients with alcohol dependence Study name Study duration Patients enrolled High drinking risk 24 -week 604 (306+298) 350 180+170 24 -week 718 (358+360) 317 (155+162) 187 52 -week 675 (509+166) ESENSE 1 (Mann 2013; Wim van den Brink 2013) ESENSE 2 (Gual 2013; Wim van den Brink 2013) SENSE (Wim van den Brink 2014) Mann et al. 2013. Biol Psychiatry 73(8) 706 -713 Gual et al. 2013. Eur Neuropsychopharmacol 23(11) 1432 -42 Wim van den Brink et al. 2014. J Psychopharmacol Wim van den Brink et al. 2013. Alcohol and Alcoholism. 1– 9 11

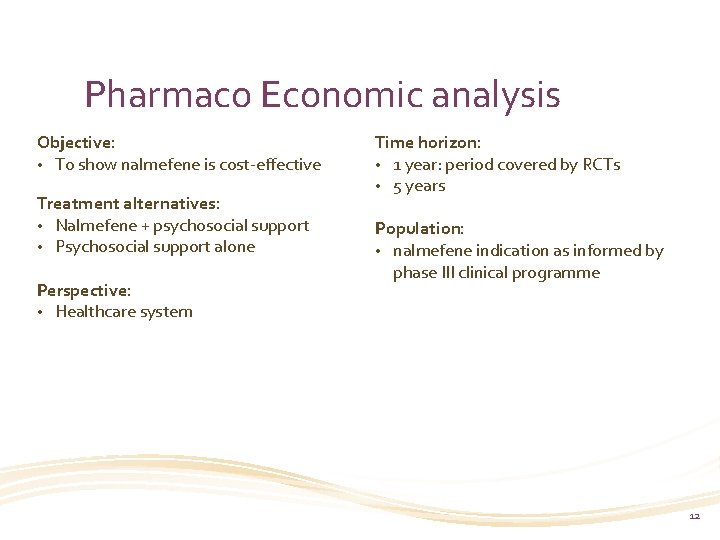
Pharmaco Economic analysis Objective: • To show nalmefene is cost-effective Treatment alternatives: • Nalmefene + psychosocial support • Psychosocial support alone Perspective: • Healthcare system Time horizon: • 1 year: period covered by RCTs • 5 years Population: • nalmefene indication as informed by phase III clinical programme 12

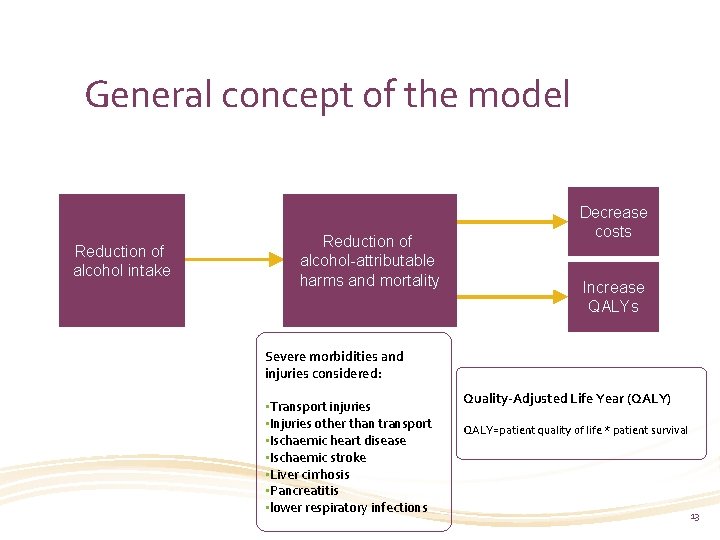
General concept of the model Reduction of alcohol intake Reduction of alcohol-attributable harms and mortality Decrease costs Increase QALYs Severe morbidities and injuries considered: • Transport injuries • Injuries other than transport • Ischaemic heart disease • Ischaemic stroke • Liver cirrhosis • Pancreatitis • lower respiratory infections Quality-Adjusted Life Year (QALY) QALY=patient quality of life * patient survival 13

Clarification questions 14

1. Patient discontinuation 2. Calculation of number of days taking therapy 3. Application of utility in the model 4. Proportion who receive care at a specialist level 5. Real world discussion of relapse rate 15

Final recommendation 16

17

Questions….