EXAMPLE OF DRUG DEVELOPMENT 2 Objectives of preclinical

EXAMPLE OF DRUG DEVELOPMENT
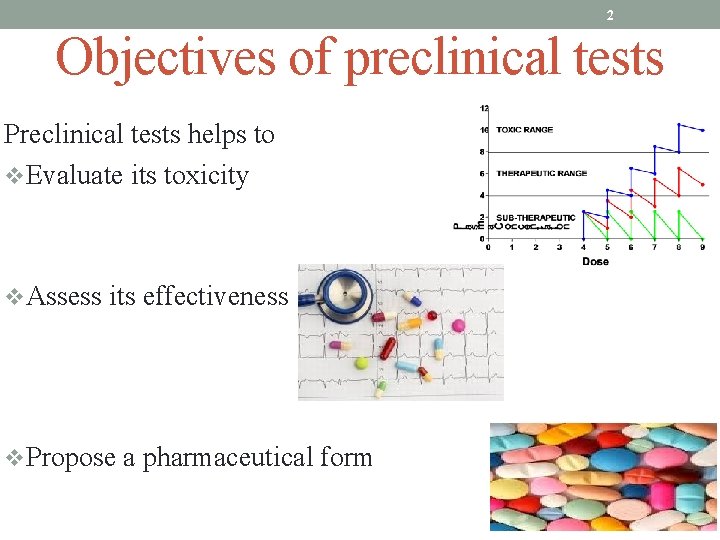
2 Objectives of preclinical tests Preclinical tests helps to v. Evaluate its toxicity v. Assess its effectiveness v. Propose a pharmaceutical form

3 Objectives (2) Preclinical studies: v. Select appropriate models based on target and Mode of Action v. These studies can: v. Provide nonclinical proof of principle regarding mechanism of action and efficacy v. Guide schedule and dose escalation schemes v. Provide information for selection of test species v. Aid in start dose selection v. Selection of investigations biomarkers : The identification of pathways involved in the mechanism of action is also essential for the selection of biomarkers of the biological activity which can be used clinically for the optimization of dosages and treatment regimens. v. Justify pharmaceutical combinations v. Understand pharmacodynamic properties
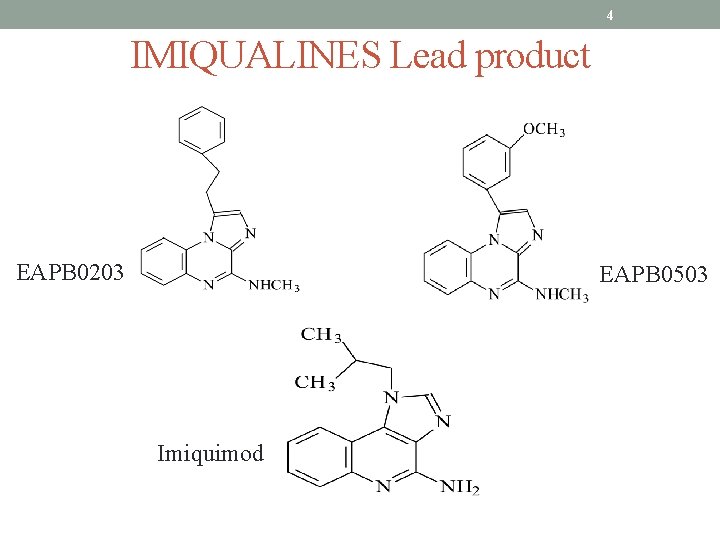
4 IMIQUALINES Lead product EAPB 0203 EAPB 0503 Imiquimod
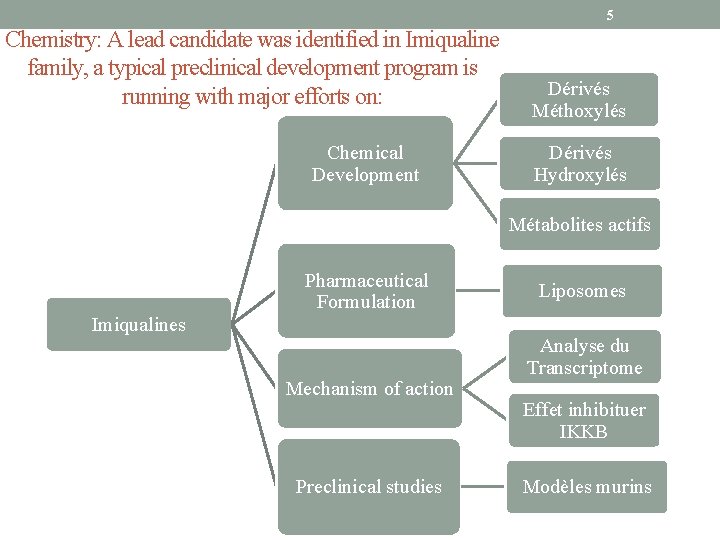
5 Chemistry: A lead candidate was identified in Imiqualine family, a typical preclinical development program is running with major efforts on: Chemical Development Dérivés Méthoxylés Dérivés Hydroxylés Métabolites actifs Pharmaceutical Formulation Imiqualines Mechanism of action Preclinical studies Liposomes Analyse du Transcriptome Effet inhibituer IKKB Modèles murins
6 Chemical level Starting in laboratory synthesis scale, then synthesis in semipilot scale

7 Pharmaceutical development

8 Pre-formulation
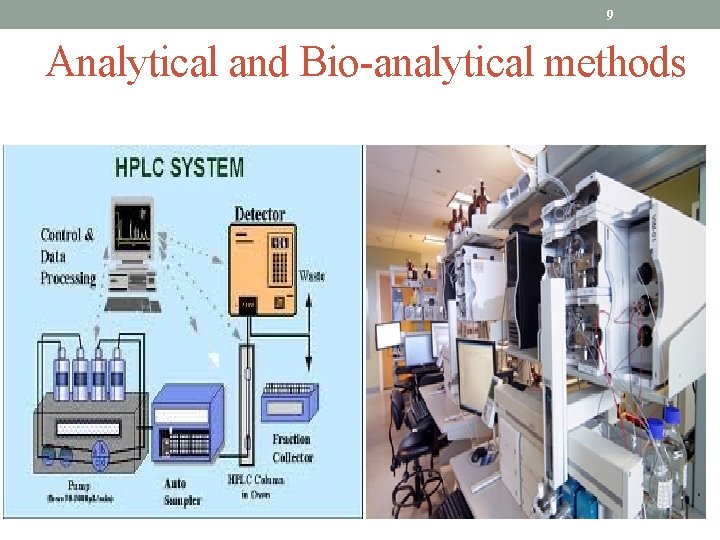
9 Analytical and Bio-analytical methods
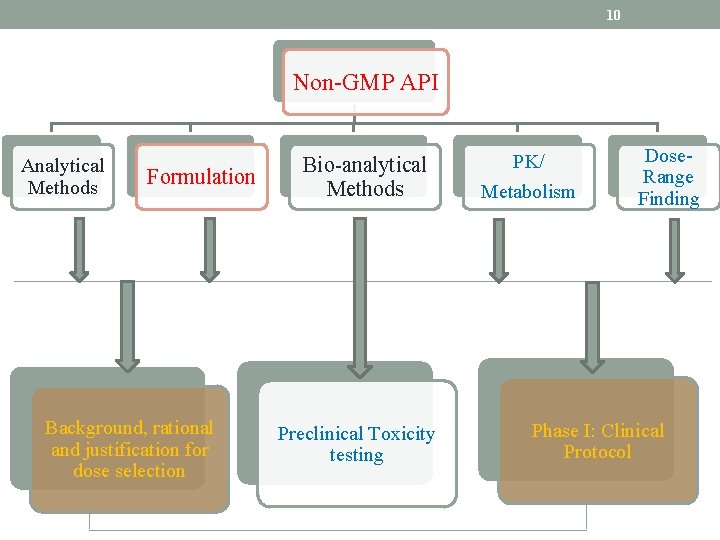
10 Non-GMP API Analytical Methods Formulation Background, rational and justification for dose selection Bio-analytical Methods Preclinical Toxicity testing PK/ Metabolism Dose. Range Finding Phase I: Clinical Protocol
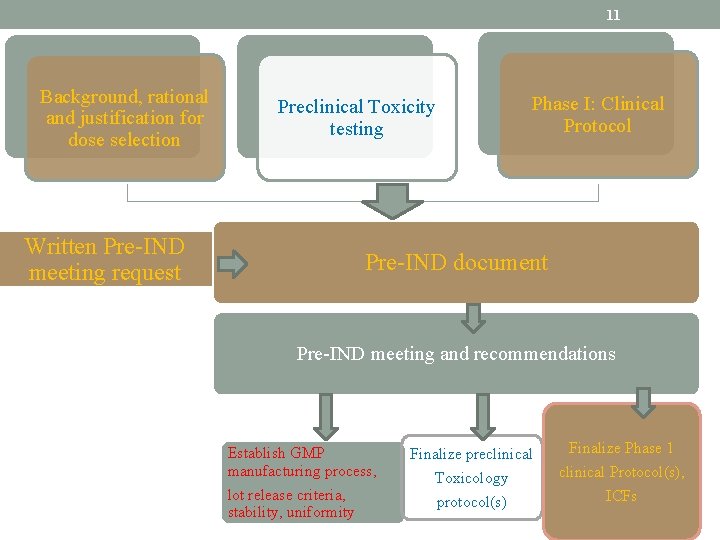
11 Background, rational and justification for dose selection Preclinical Toxicity testing Written Pre-IND meeting request Phase I: Clinical Protocol Pre-IND document Pre-IND meeting and recommendations Establish GMP manufacturing process, lot release criteria, stability, uniformity Finalize preclinical Toxicology protocol(s) Finalize Phase 1 clinical Protocol(s), ICFs

PHARMACOLOGY TEST EXAMPLE OF ANTICANCER DRUG
13 Cancer cell lines v. In vitro studies performed in cell lines, cell-free systems v. Often form the basis for screening and optimization during discovery v. Animal screening is too expensive for routine use v. Cellular uptake and membrane transport v. MDR, MRP, etc v. Predictions of bioavailability and distribution v. In vitro drug metabolism: v. P 450 isoenzyme inhibition or induction v. Effects on h. ERG channels (prolonged QT interval risk) v. Preliminary protein binding studies

14 The NCI-60: Assessing drug effectiveness NCI's In Vitro Cell Line Screening Project, better known as the NCI-60 analyzes the anti-cancer properties of a compound in human tumor samples from 60 different cell cultures, sometimes referred to as lines, representing leukemia, melanoma, and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney. The NCI-60 project, which has been testing lines since 1990 in the Developmental Therapeutics Program of NCI's Division of Cancer Treatment and Diagnosis, screened 17, 200 compounds in 2011, roughly evenly divided between natural and synthetic agents. The most promising—the "hits"—move on to further testing. Since 1990, more than 100, 000 natural products have gone through the NCI screening process, driving the number of drugs in NCI's repository that have had some kind of screening process to over 400, 000. There is no cost to the researcher for NCI-60 screening.
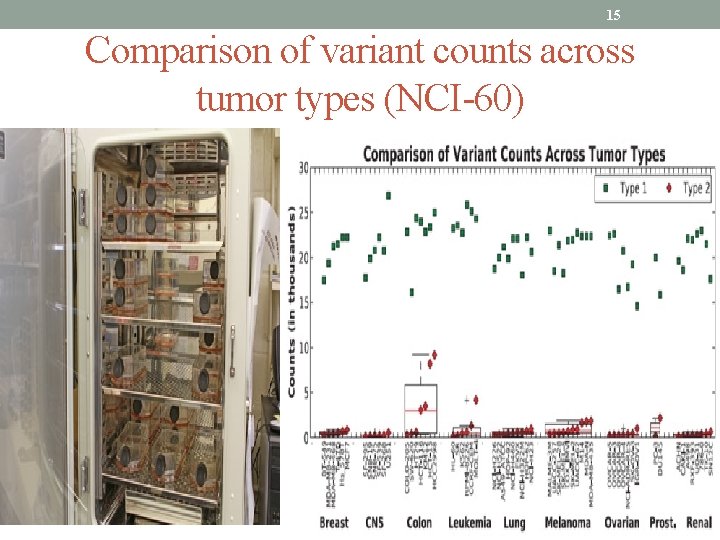
15 Comparison of variant counts across tumor types (NCI-60)
16 Animal models v. Efficacy demonstrated in disease specific animal models: v. Proof of therapeutic principle v. Groundwork for clinical development planning v. Evaluation of therapeutic index v. Toxicity versus efficacy
17 Animal models (2) v. Ideal Animal Model v. Validity v. Selectivity v. Predictability v. Reproducibility
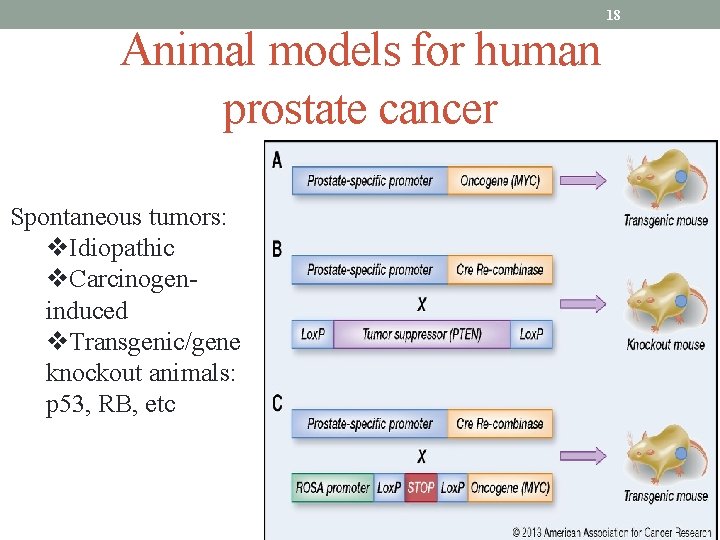
Animal models for human prostate cancer Spontaneous tumors: v. Idiopathic v. Carcinogeninduced v. Transgenic/gene knockout animals: p 53, RB, etc 18

19 Animal Pharmacokinetic models v. Animal pharmacokinetics can guide dose and schedule selection v. ADME data can be generated in parallel with clinical development v. Preliminary evaluation of candidate biomarkers

20 Cage à Métabolisme
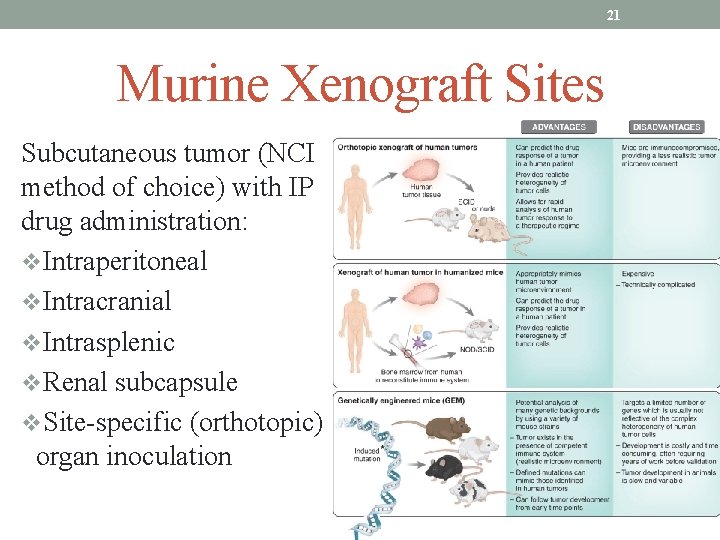
21 Murine Xenograft Sites Subcutaneous tumor (NCI method of choice) with IP drug administration: v. Intraperitoneal v. Intracranial v. Intrasplenic v. Renal subcapsule v. Site-specific (orthotopic) organ inoculation

22 Xenograft Advantages Many different human tumor cell lines transplantable Wide representation of most human solid tumors Allows for evaluation of therapeutic index Good correlation with drug regimens active in human lung, colon, breast, and melanoma cancers Several decades of experience Disadvantages Brain tumors difficult to model Metastases rare Survival not an ideal endpoint: death from bulk of tumor, not invasion Shorter doubling times than original growth in human Less necrosis, better blood supply Difficult to maintain animals due to infection risks Ability to mimic the human tumor microenvironment is limited

23 Animal models (3) There is no perfect tumor model How Predictive is the disease model in comparison to human disease ? ? ?
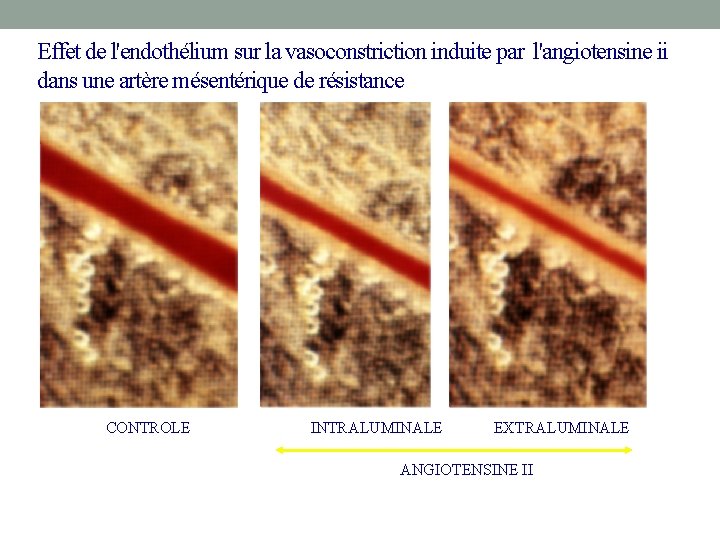
Effet de l'endothélium sur la vasoconstriction induite par l'angiotensine ii dans une artère mésentérique de résistance CONTROLE INTRALUMINALE EXTRALUMINALE ANGIOTENSINE II
- Slides: 24