Example Exercise 12 5 Electron Dot FormulasSingle Bonds
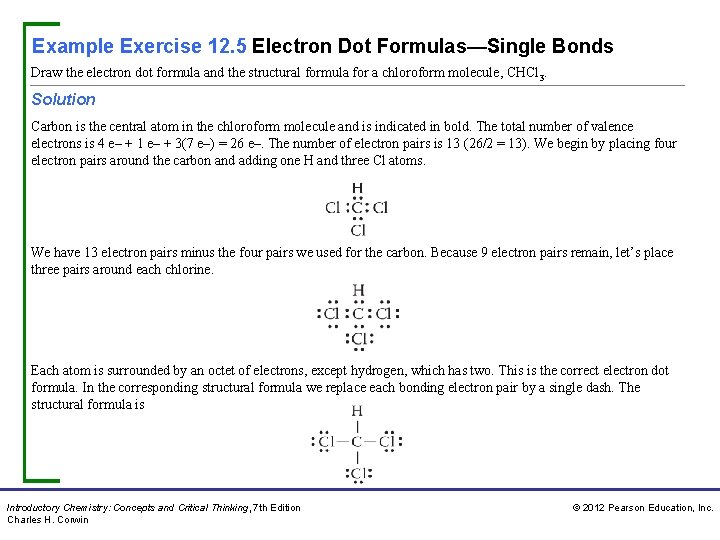
Example Exercise 12. 5 Electron Dot Formulas—Single Bonds Draw the electron dot formula and the structural formula for a chloroform molecule, CHCl 3. Solution Carbon is the central atom in the chloroform molecule and is indicated in bold. The total number of valence electrons is 4 e– + 1 e– + 3(7 e–) = 26 e–. The number of electron pairs is 13 (26/2 = 13). We begin by placing four electron pairs around the carbon and adding one H and three Cl atoms. We have 13 electron pairs minus the four pairs we used for the carbon. Because 9 electron pairs remain, let’s place three pairs around each chlorine. Each atom is surrounded by an octet of electrons, except hydrogen, which has two. This is the correct electron dot formula. In the corresponding structural formula we replace each bonding electron pair by a single dash. The structural formula is Introductory Chemistry: Concepts and Critical Thinking, 7 th Edition Charles H. Corwin © 2012 Pearson Education, Inc.
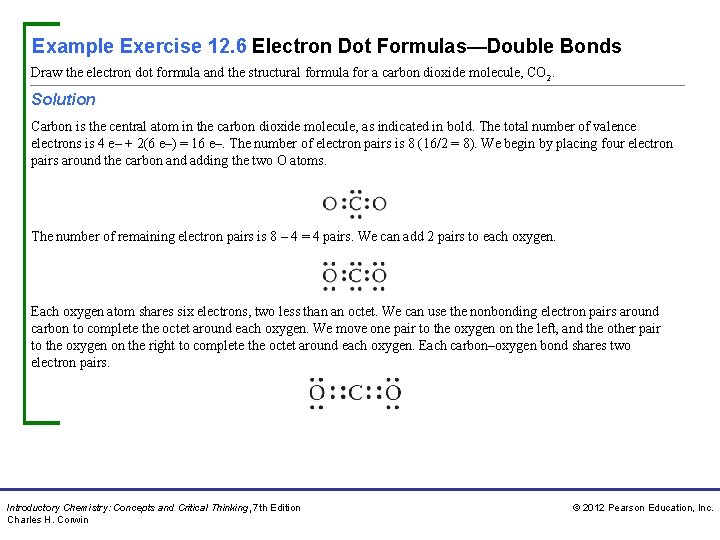
Example Exercise 12. 6 Electron Dot Formulas—Double Bonds Draw the electron dot formula and the structural formula for a carbon dioxide molecule, CO 2. Solution Carbon is the central atom in the carbon dioxide molecule, as indicated in bold. The total number of valence electrons is 4 e– + 2(6 e–) = 16 e–. The number of electron pairs is 8 (16/2 = 8). We begin by placing four electron pairs around the carbon and adding the two O atoms. The number of remaining electron pairs is 8 – 4 = 4 pairs. We can add 2 pairs to each oxygen. Each oxygen atom shares six electrons, two less than an octet. We can use the nonbonding electron pairs around carbon to complete the octet around each oxygen. We move one pair to the oxygen on the left, and the other pair to the oxygen on the right to complete the octet around each oxygen. Each carbon–oxygen bond shares two electron pairs. Introductory Chemistry: Concepts and Critical Thinking, 7 th Edition Charles H. Corwin © 2012 Pearson Education, Inc.
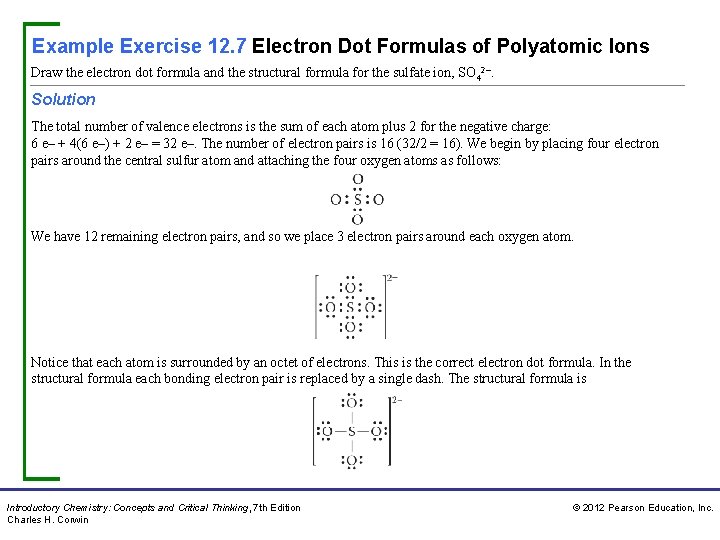
Example Exercise 12. 7 Electron Dot Formulas of Polyatomic Ions Draw the electron dot formula and the structural formula for the sulfate ion, SO 42–. Solution The total number of valence electrons is the sum of each atom plus 2 for the negative charge: 6 e– + 4(6 e–) + 2 e– = 32 e–. The number of electron pairs is 16 (32/2 = 16). We begin by placing four electron pairs around the central sulfur atom and attaching the four oxygen atoms as follows: We have 12 remaining electron pairs, and so we place 3 electron pairs around each oxygen atom. Notice that each atom is surrounded by an octet of electrons. This is the correct electron dot formula. In the structural formula each bonding electron pair is replaced by a single dash. The structural formula is Introductory Chemistry: Concepts and Critical Thinking, 7 th Edition Charles H. Corwin © 2012 Pearson Education, Inc.
- Slides: 3