EXAMPLE 7 1 Evidence of a Chemical Reaction




- Slides: 33

EXAMPLE 7. 1 Evidence of a Chemical Reaction Which of the following is a chemical reaction? Why? (a) ice melting upon warming (b) an electric current is passed through water, resulting in the formation of hydrogen and oxygen gas that appear as bubbles rising in the water (c) iron rusting (d) bubbles forming when a soda can is opened Solution: (a) Not a chemical reaction; melting ice forms water, but both the ice and water are composed of water molecules. (b) Chemical reaction; water decomposes into hydrogen and oxygen, as evidenced by the bubbling. (c) Chemical reaction; iron changes into iron oxide, changing color in the process. (d) Not a chemical reaction; even though there is bubbling, it is just carbon dioxide coming out of the liquid. SKILLBUILDER 7. 1 Evidence of a Chemical Reaction Which of the following is a chemical reaction? Why? (a) butane burning in a butane lighter (b) butane evaporating out of a butane lighter (c) wood burning (d) dry ice subliming FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 16; Problems 29, 30, 31, 32, 33, 34. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

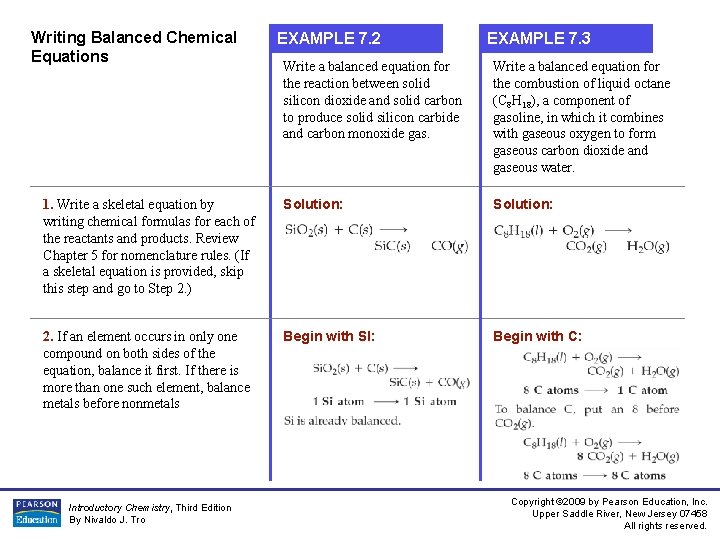
Writing Balanced Chemical Equations EXAMPLE 7. 2 EXAMPLE 7. 3 Write a balanced equation for the reaction between solid silicon dioxide and solid carbon to produce solid silicon carbide and carbon monoxide gas. Write a balanced equation for the combustion of liquid octane (C 8 H 18), a component of gasoline, in which it combines with gaseous oxygen to form gaseous carbon dioxide and gaseous water. 1. Write a skeletal equation by writing chemical formulas for each of the reactants and products. Review Chapter 5 for nomenclature rules. (If a skeletal equation is provided, skip this step and go to Step 2. ) Solution: 2. If an element occurs in only one compound on both sides of the equation, balance it first. If there is more than one such element, balance metals before nonmetals Begin with Sl: Begin with C: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

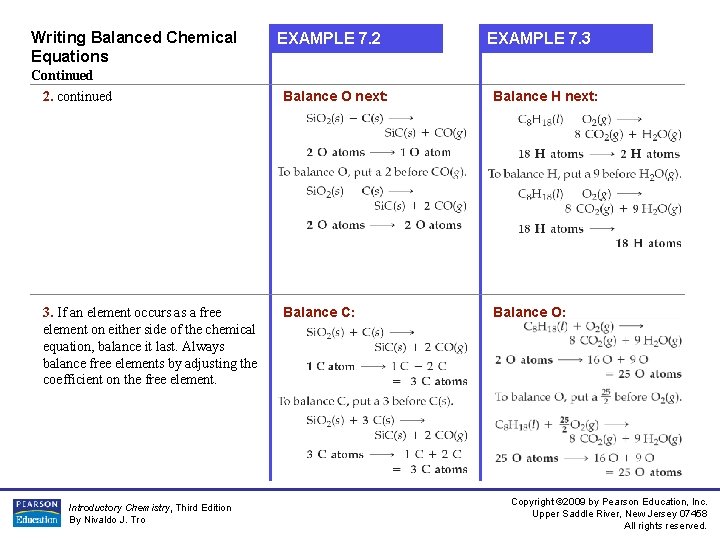
Writing Balanced Chemical Equations EXAMPLE 7. 2 EXAMPLE 7. 3 Continued 2. continued Balance O next: Balance H next: 3. If an element occurs as a free element on either side of the chemical equation, balance it last. Always balance free elements by adjusting the coefficient on the free element. Balance C: Balance O: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

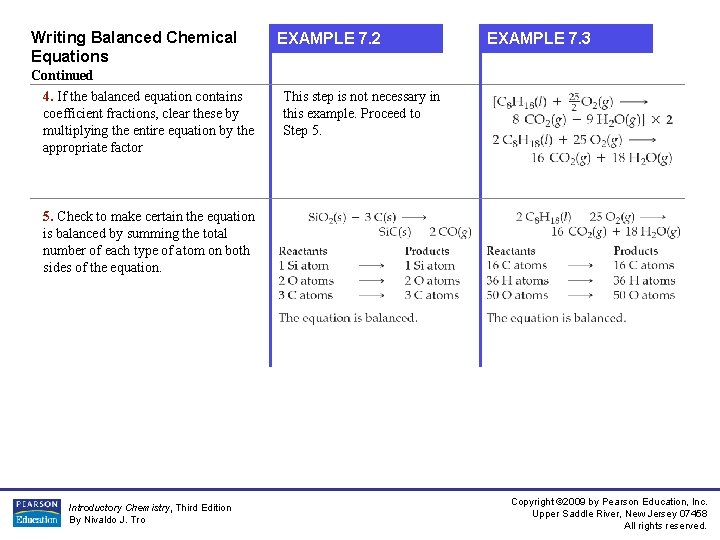
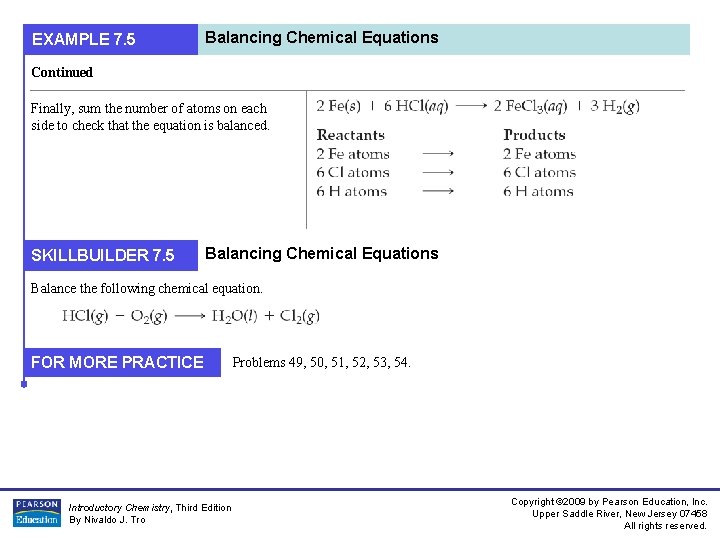
Writing Balanced Chemical Equations EXAMPLE 7. 2 EXAMPLE 7. 3 Continued 4. If the balanced equation contains coefficient fractions, clear these by multiplying the entire equation by the appropriate factor This step is not necessary in this example. Proceed to Step 5. Check to make certain the equation is balanced by summing the total number of each type of atom on both sides of the equation. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

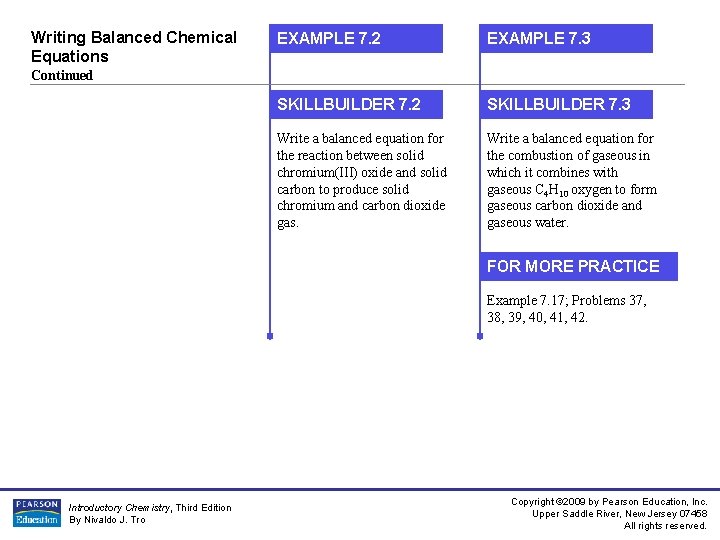
Writing Balanced Chemical Equations EXAMPLE 7. 2 EXAMPLE 7. 3 SKILLBUILDER 7. 2 SKILLBUILDER 7. 3 Write a balanced equation for the reaction between solid chromium(III) oxide and solid carbon to produce solid chromium and carbon dioxide gas. Write a balanced equation for the combustion of gaseous in which it combines with gaseous C 4 H 10 oxygen to form gaseous carbon dioxide and gaseous water. Continued FOR MORE PRACTICE Example 7. 17; Problems 37, 38, 39, 40, 41, 42. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

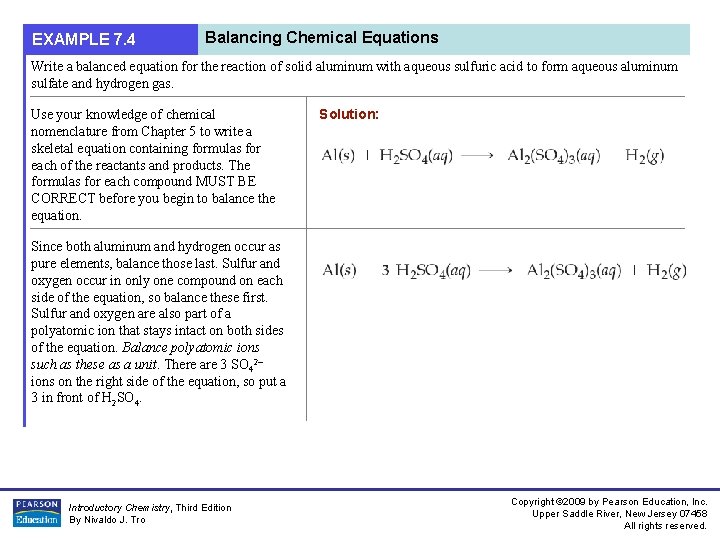
EXAMPLE 7. 4 Balancing Chemical Equations Write a balanced equation for the reaction of solid aluminum with aqueous sulfuric acid to form aqueous aluminum sulfate and hydrogen gas. Use your knowledge of chemical nomenclature from Chapter 5 to write a skeletal equation containing formulas for each of the reactants and products. The formulas for each compound MUST BE CORRECT before you begin to balance the equation. Solution: Since both aluminum and hydrogen occur as pure elements, balance those last. Sulfur and oxygen occur in only one compound on each side of the equation, so balance these first. Sulfur and oxygen are also part of a polyatomic ion that stays intact on both sides of the equation. Balance polyatomic ions such as these as a unit. There are 3 SO 42– ions on the right side of the equation, so put a 3 in front of H 2 SO 4. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

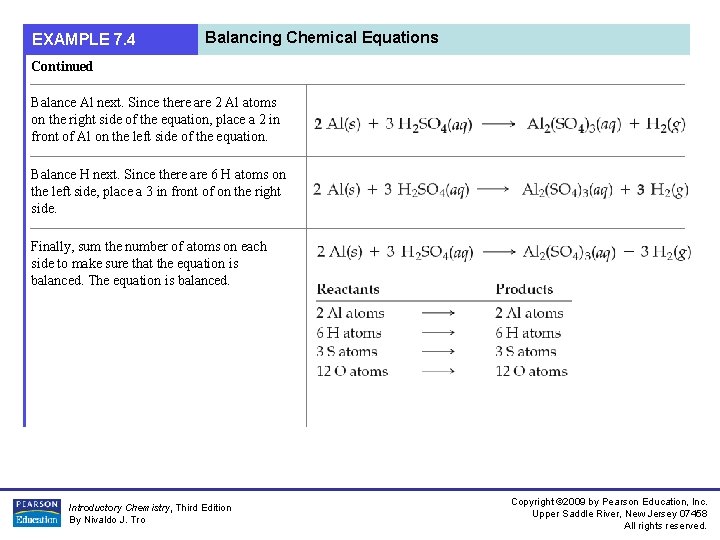
EXAMPLE 7. 4 Balancing Chemical Equations Continued Balance Al next. Since there are 2 Al atoms on the right side of the equation, place a 2 in front of Al on the left side of the equation. Balance H next. Since there are 6 H atoms on the left side, place a 3 in front of on the right side. Finally, sum the number of atoms on each side to make sure that the equation is balanced. The equation is balanced. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

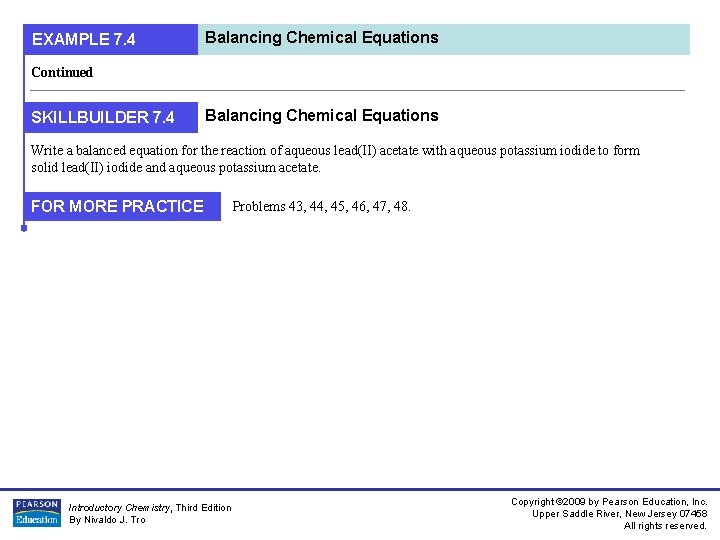
EXAMPLE 7. 4 Balancing Chemical Equations Continued SKILLBUILDER 7. 4 Balancing Chemical Equations Write a balanced equation for the reaction of aqueous lead(II) acetate with aqueous potassium iodide to form solid lead(II) iodide and aqueous potassium acetate. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 43, 44, 45, 46, 47, 48. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

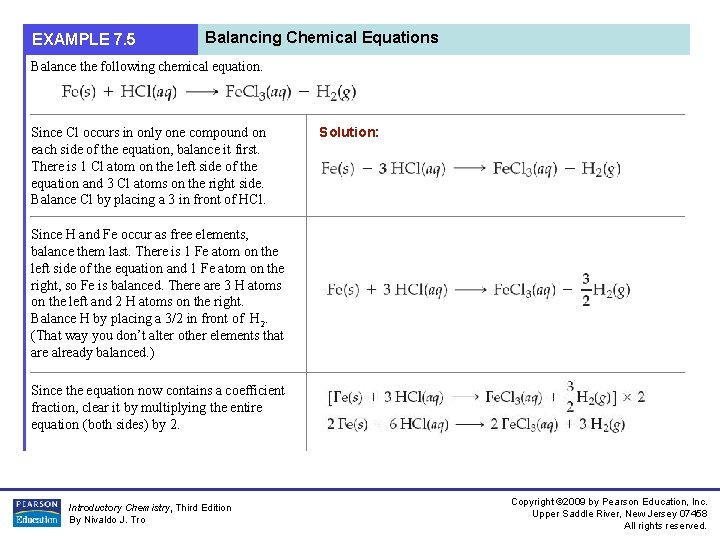
EXAMPLE 7. 5 Balancing Chemical Equations Balance the following chemical equation. Since Cl occurs in only one compound on each side of the equation, balance it first. There is 1 Cl atom on the left side of the equation and 3 Cl atoms on the right side. Balance Cl by placing a 3 in front of HCl. Solution: Since H and Fe occur as free elements, balance them last. There is 1 Fe atom on the left side of the equation and 1 Fe atom on the right, so Fe is balanced. There are 3 H atoms on the left and 2 H atoms on the right. Balance H by placing a 3/2 in front of H 2. (That way you don’t alter other elements that are already balanced. ) Since the equation now contains a coefficient fraction, clear it by multiplying the entire equation (both sides) by 2. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 7. 5 Balancing Chemical Equations Continued Finally, sum the number of atoms on each side to check that the equation is balanced. SKILLBUILDER 7. 5 Balancing Chemical Equations Balance the following chemical equation. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 49, 50, 51, 52, 53, 54. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 7. 6 Determining Whether a Compound Is Soluble Determine whether each of the following compounds is soluble or insoluble. (a) Ag. Br (b) Ca. Cl 2 (c) Pb(NO 3)2 (d) Pb. SO 4 Solution: (a) Insoluble; compounds containing Br– are normally soluble, but Ag+ is an exception. (b) Soluble; compounds containing Cl– are normally soluble, and Ca 2– is not an exception. (c) Soluble; compounds containing NO 3– are always soluble. (d) Insoluble; compounds containing SO 42– are normally soluble, but PB 2+ is an exception. SKILLBUILDER 7. 6 Determining Whether a Compound Is Soluble Determine whether each of the following compounds is soluble or insoluble. (a) Cu. S (b) Fe. SO 4 (c) Pb. CO 3 (d) NH 4 Cl FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 18; Problems 61, 62, 63, 64, 65, 66. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

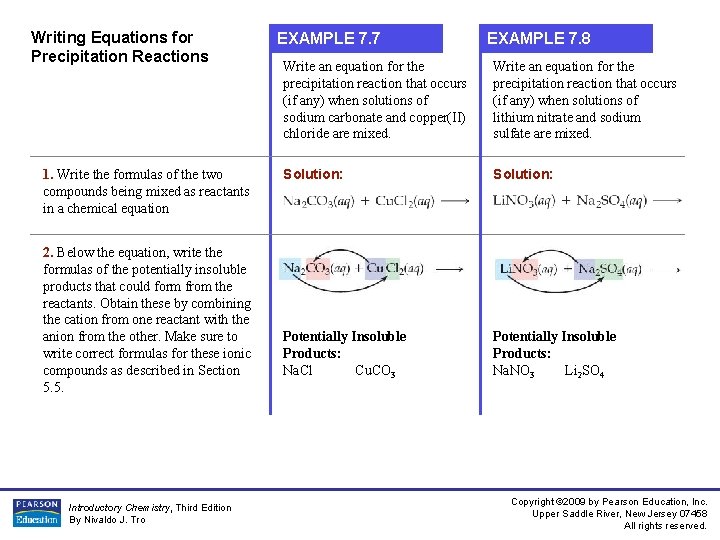
Writing Equations for Precipitation Reactions 1. Write the formulas of the two compounds being mixed as reactants in a chemical equation 2. Below the equation, write the formulas of the potentially insoluble products that could form from the reactants. Obtain these by combining the cation from one reactant with the anion from the other. Make sure to write correct formulas for these ionic compounds as described in Section 5. 5. Introductory Chemistry, Third Edition By Nivaldo J. Tro EXAMPLE 7. 7 EXAMPLE 7. 8 Write an equation for the precipitation reaction that occurs (if any) when solutions of sodium carbonate and copper(II) chloride are mixed. Write an equation for the precipitation reaction that occurs (if any) when solutions of lithium nitrate and sodium sulfate are mixed. Solution: Potentially Insoluble Products: Na. Cl Cu. CO 3 Potentially Insoluble Products: Na. NO 3 Li 2 SO 4 Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

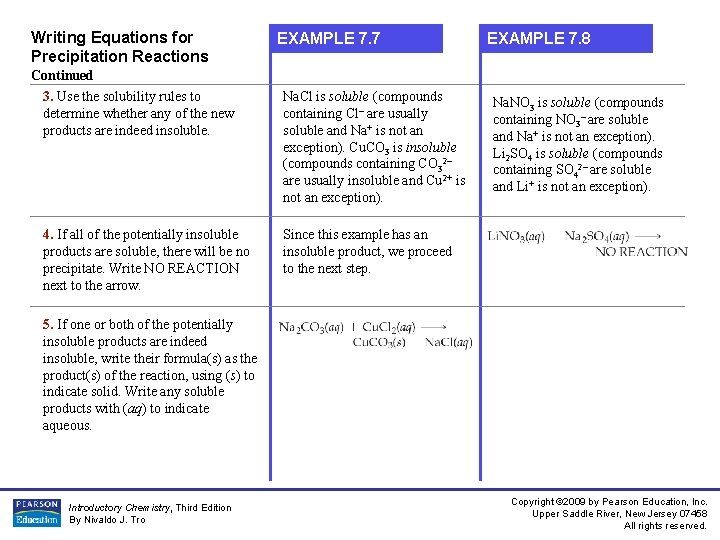
Writing Equations for Precipitation Reactions EXAMPLE 7. 7 EXAMPLE 7. 8 Continued 3. Use the solubility rules to determine whether any of the new products are indeed insoluble. Na. Cl is soluble (compounds containing Cl– are usually soluble and Na+ is not an exception). Cu. CO 3 is insoluble (compounds containing CO 32– are usually insoluble and Cu 2+ is not an exception). 4. If all of the potentially insoluble products are soluble, there will be no precipitate. Write NO REACTION next to the arrow. Since this example has an insoluble product, we proceed to the next step. Na. NO 3 is soluble (compounds containing NO 3– are soluble and Na+ is not an exception). Li 2 SO 4 is soluble (compounds containing SO 42– are soluble and Li+ is not an exception). 5. If one or both of the potentially insoluble products are indeed insoluble, write their formula(s) as the product(s) of the reaction, using (s) to indicate solid. Write any soluble products with (aq) to indicate aqueous. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

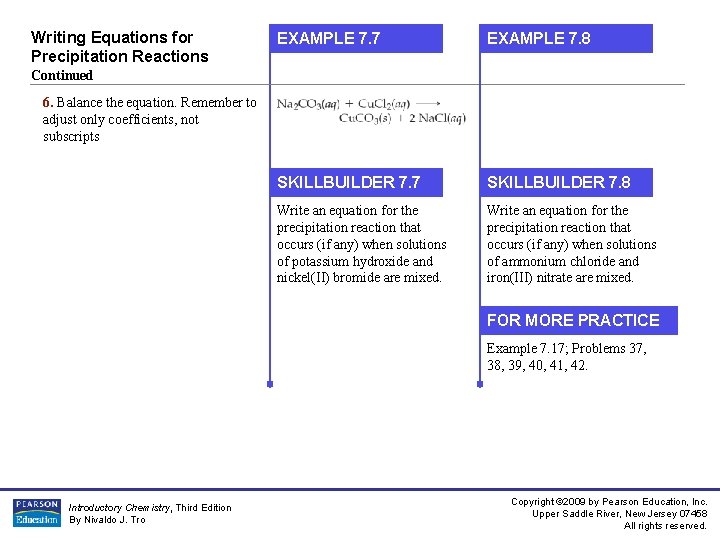
Writing Equations for Precipitation Reactions EXAMPLE 7. 7 EXAMPLE 7. 8 SKILLBUILDER 7. 7 SKILLBUILDER 7. 8 Write an equation for the precipitation reaction that occurs (if any) when solutions of potassium hydroxide and nickel(II) bromide are mixed. Write an equation for the precipitation reaction that occurs (if any) when solutions of ammonium chloride and iron(III) nitrate are mixed. Continued 6. Balance the equation. Remember to adjust only coefficients, not subscripts FOR MORE PRACTICE Example 7. 17; Problems 37, 38, 39, 40, 41, 42. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

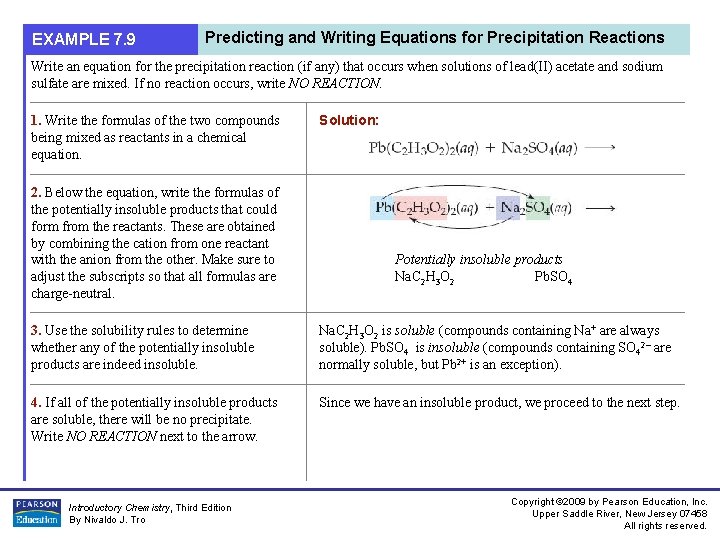
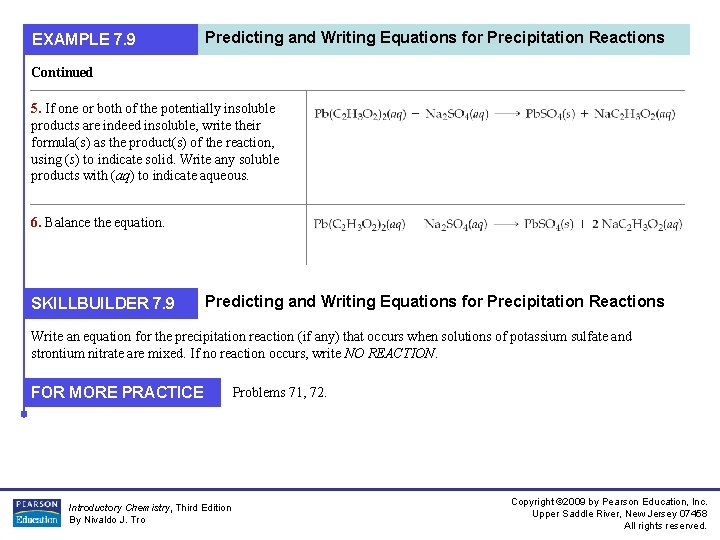
EXAMPLE 7. 9 Predicting and Writing Equations for Precipitation Reactions Write an equation for the precipitation reaction (if any) that occurs when solutions of lead(II) acetate and sodium sulfate are mixed. If no reaction occurs, write NO REACTION. 1. Write the formulas of the two compounds being mixed as reactants in a chemical equation. 2. Below the equation, write the formulas of the potentially insoluble products that could form from the reactants. These are obtained by combining the cation from one reactant with the anion from the other. Make sure to adjust the subscripts so that all formulas are charge-neutral. Solution: Potentially insoluble products Na. C 2 H 3 O 2 Pb. SO 4 3. Use the solubility rules to determine whether any of the potentially insoluble products are indeed insoluble. Na. C 2 H 3 O 2 is soluble (compounds containing Na+ are always soluble). Pb. SO 4 is insoluble (compounds containing SO 42– are normally soluble, but Pb 2+ is an exception). 4. If all of the potentially insoluble products are soluble, there will be no precipitate. Write NO REACTION next to the arrow. Since we have an insoluble product, we proceed to the next step. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 7. 9 Predicting and Writing Equations for Precipitation Reactions Continued 5. If one or both of the potentially insoluble products are indeed insoluble, write their formula(s) as the product(s) of the reaction, using (s) to indicate solid. Write any soluble products with (aq) to indicate aqueous. 6. Balance the equation. SKILLBUILDER 7. 9 Predicting and Writing Equations for Precipitation Reactions Write an equation for the precipitation reaction (if any) that occurs when solutions of potassium sulfate and strontium nitrate are mixed. If no reaction occurs, write NO REACTION. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 71, 72. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

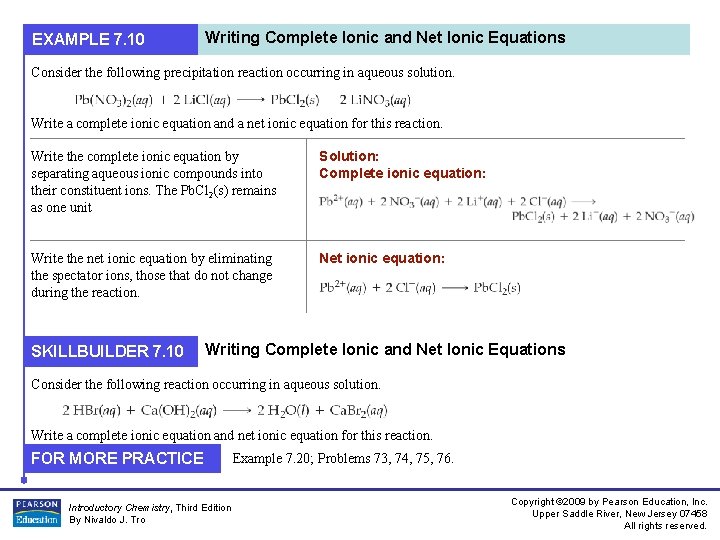
EXAMPLE 7. 10 Writing Complete Ionic and Net Ionic Equations Consider the following precipitation reaction occurring in aqueous solution. Write a complete ionic equation and a net ionic equation for this reaction. Write the complete ionic equation by separating aqueous ionic compounds into their constituent ions. The Pb. Cl 2(s) remains as one unit Solution: Complete ionic equation: Write the net ionic equation by eliminating the spectator ions, those that do not change during the reaction. Net ionic equation: SKILLBUILDER 7. 10 Writing Complete Ionic and Net Ionic Equations Consider the following reaction occurring in aqueous solution. Write a complete ionic equation and net ionic equation for this reaction. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 20; Problems 73, 74, 75, 76. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

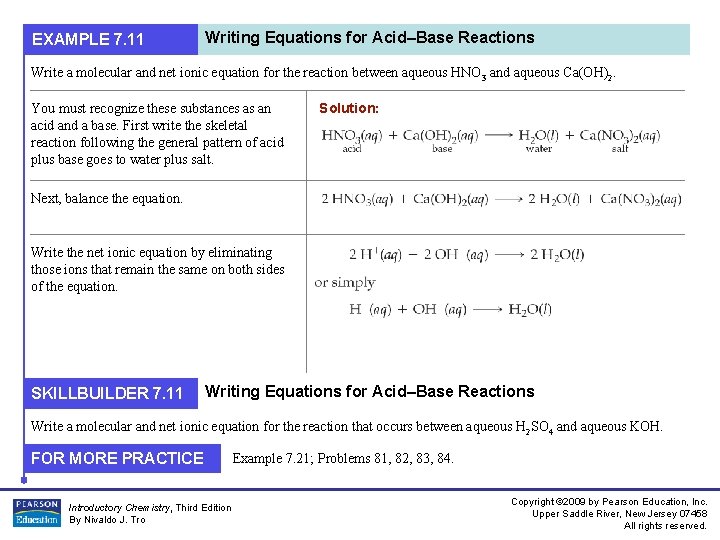
EXAMPLE 7. 11 Writing Equations for Acid–Base Reactions Write a molecular and net ionic equation for the reaction between aqueous HNO 3 and aqueous Ca(OH)2. You must recognize these substances as an acid and a base. First write the skeletal reaction following the general pattern of acid plus base goes to water plus salt. Solution: Next, balance the equation. Write the net ionic equation by eliminating those ions that remain the same on both sides of the equation. SKILLBUILDER 7. 11 Writing Equations for Acid–Base Reactions Write a molecular and net ionic equation for the reaction that occurs between aqueous H 2 SO 4 and aqueous KOH. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 21; Problems 81, 82, 83, 84. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

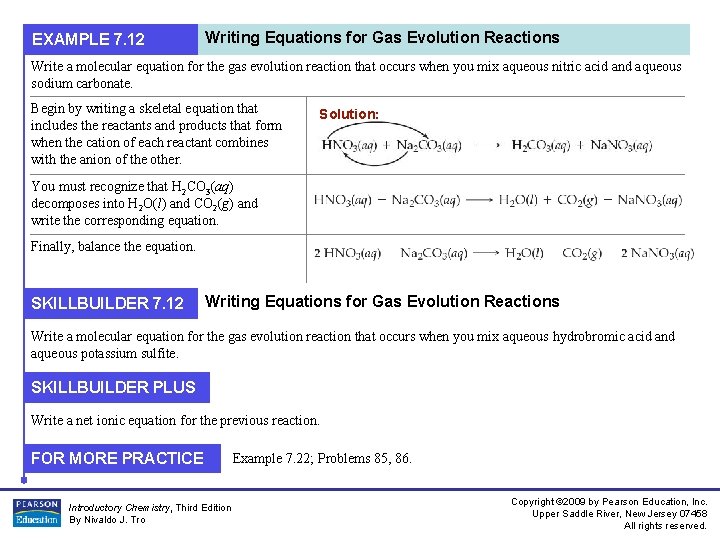
EXAMPLE 7. 12 Writing Equations for Gas Evolution Reactions Write a molecular equation for the gas evolution reaction that occurs when you mix aqueous nitric acid and aqueous sodium carbonate. Begin by writing a skeletal equation that includes the reactants and products that form when the cation of each reactant combines with the anion of the other. Solution: You must recognize that H 2 CO 3(aq) decomposes into H 2 O(l) and CO 2(g) and write the corresponding equation. Finally, balance the equation. SKILLBUILDER 7. 12 Writing Equations for Gas Evolution Reactions Write a molecular equation for the gas evolution reaction that occurs when you mix aqueous hydrobromic acid and aqueous potassium sulfite. SKILLBUILDER PLUS Write a net ionic equation for the previous reaction. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 22; Problems 85, 86. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

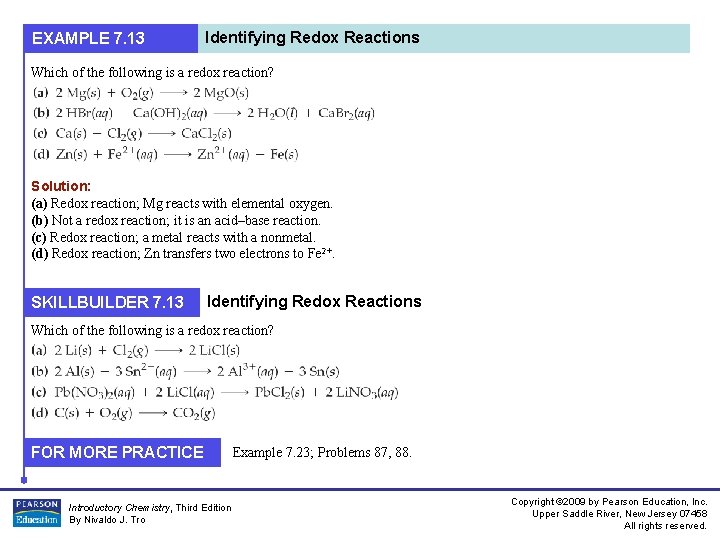
EXAMPLE 7. 13 Identifying Redox Reactions Which of the following is a redox reaction? Solution: (a) Redox reaction; Mg reacts with elemental oxygen. (b) Not a redox reaction; it is an acid–base reaction. (c) Redox reaction; a metal reacts with a nonmetal. (d) Redox reaction; Zn transfers two electrons to Fe 2+. SKILLBUILDER 7. 13 Identifying Redox Reactions Which of the following is a redox reaction? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 23; Problems 87, 88. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

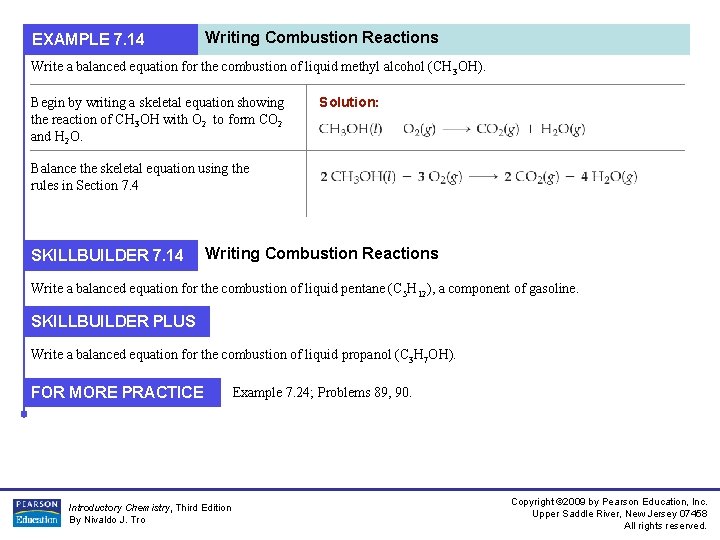
EXAMPLE 7. 14 Writing Combustion Reactions Write a balanced equation for the combustion of liquid methyl alcohol (CH 3 OH). Begin by writing a skeletal equation showing the reaction of CH 3 OH with O 2 to form CO 2 and H 2 O. Solution: Balance the skeletal equation using the rules in Section 7. 4 SKILLBUILDER 7. 14 Writing Combustion Reactions Write a balanced equation for the combustion of liquid pentane (C 5 H 12), a component of gasoline. SKILLBUILDER PLUS Write a balanced equation for the combustion of liquid propanol (C 3 H 7 OH). FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 24; Problems 89, 90. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

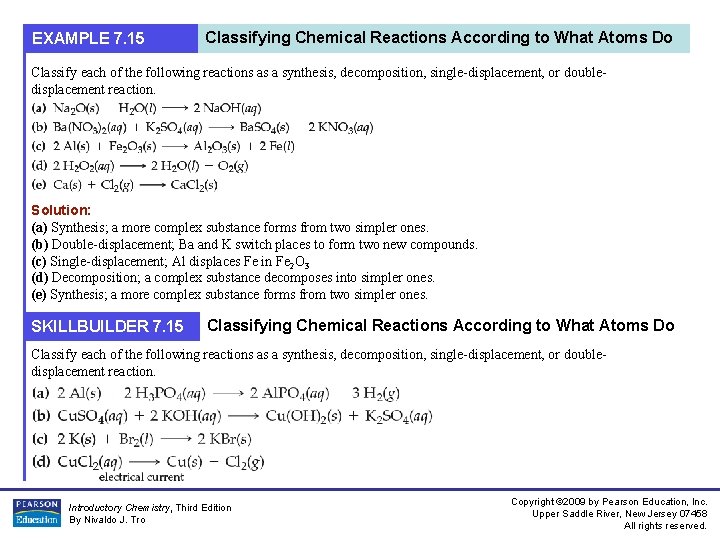
EXAMPLE 7. 15 Classifying Chemical Reactions According to What Atoms Do Classify each of the following reactions as a synthesis, decomposition, single-displacement, or doubledisplacement reaction. Solution: (a) Synthesis; a more complex substance forms from two simpler ones. (b) Double-displacement; Ba and K switch places to form two new compounds. (c) Single-displacement; Al displaces Fe in Fe 2 O 3 (d) Decomposition; a complex substance decomposes into simpler ones. (e) Synthesis; a more complex substance forms from two simpler ones. SKILLBUILDER 7. 15 Classifying Chemical Reactions According to What Atoms Do Classify each of the following reactions as a synthesis, decomposition, single-displacement, or doubledisplacement reaction. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 7. 15 Classifying Chemical Reactions According to What Atoms Do Continued FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 7. 25; Problems 93, 94, 95, 96. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

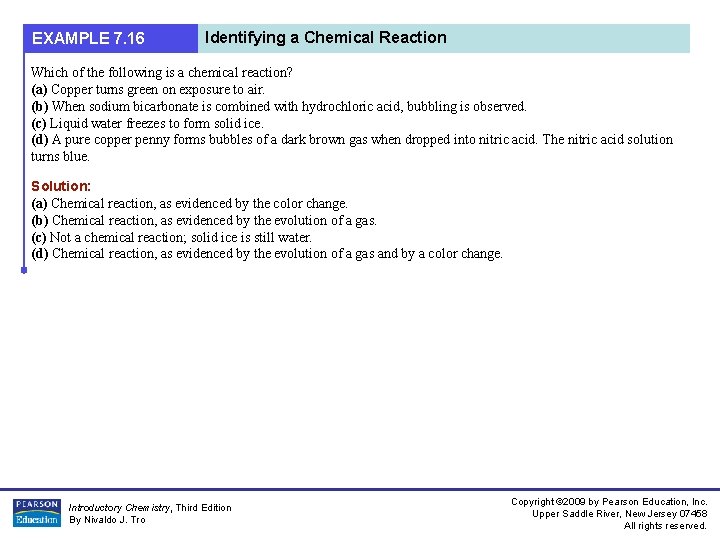
EXAMPLE 7. 16 Identifying a Chemical Reaction Which of the following is a chemical reaction? (a) Copper turns green on exposure to air. (b) When sodium bicarbonate is combined with hydrochloric acid, bubbling is observed. (c) Liquid water freezes to form solid ice. (d) A pure copper penny forms bubbles of a dark brown gas when dropped into nitric acid. The nitric acid solution turns blue. Solution: (a) Chemical reaction, as evidenced by the color change. (b) Chemical reaction, as evidenced by the evolution of a gas. (c) Not a chemical reaction; solid ice is still water. (d) Chemical reaction, as evidenced by the evolution of a gas and by a color change. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 7. 17 Writing Balanced Chemical Equations Write a balanced chemical equation for the reaction of solid vanadium(V) oxide with hydrogen gas to form solid vanadium(III) oxide and liquid water. Solution: Vanadium occurs in only one compound on both sides of the equation. However, it is balanced, so we proceed to balance oxygen by placing a 2 in front of H 2 O on the right side. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

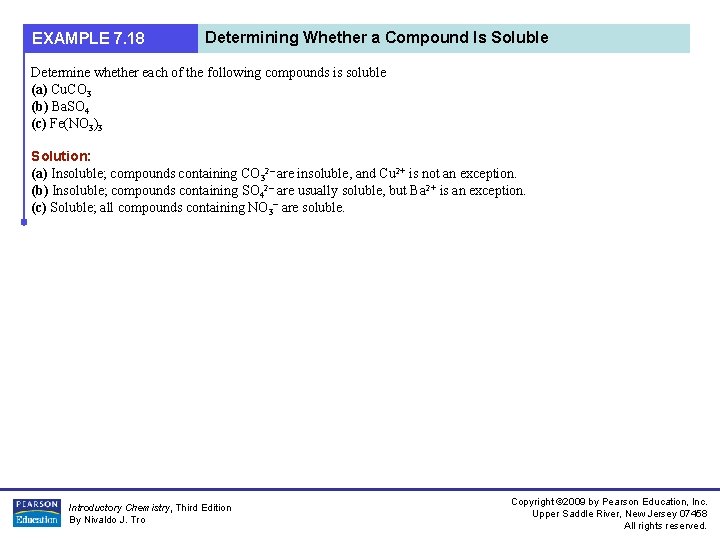
EXAMPLE 7. 18 Determining Whether a Compound Is Soluble Determine whether each of the following compounds is soluble (a) Cu. CO 3 (b) Ba. SO 4 (c) Fe(NO 3)3 Solution: (a) Insoluble; compounds containing CO 32– are insoluble, and Cu 2+ is not an exception. (b) Insoluble; compounds containing SO 42– are usually soluble, but Ba 2+ is an exception. (c) Soluble; all compounds containing NO 3– are soluble. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

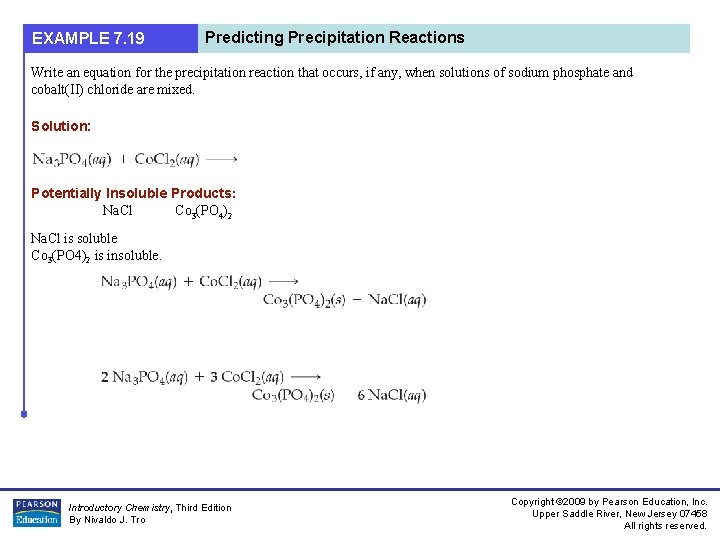
EXAMPLE 7. 19 Predicting Precipitation Reactions Write an equation for the precipitation reaction that occurs, if any, when solutions of sodium phosphate and cobalt(II) chloride are mixed. Solution: Potentially Insoluble Products: Na. Cl Co 3(PO 4)2 Na. Cl is soluble Co 3(PO 4)2 is insoluble. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

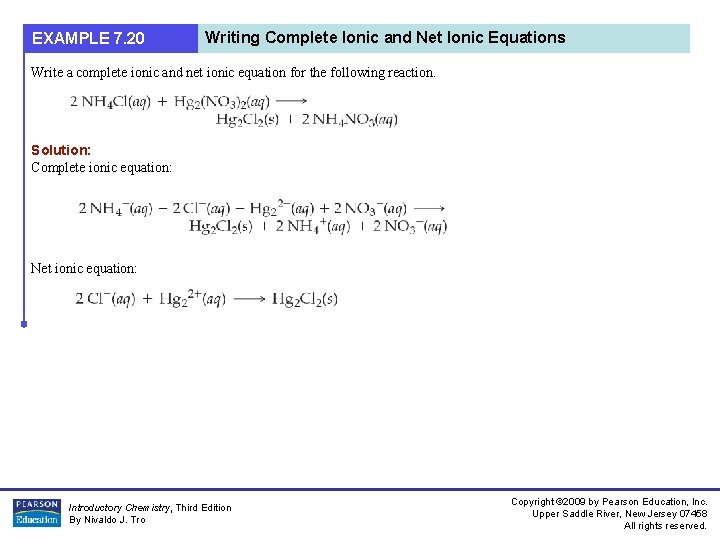
EXAMPLE 7. 20 Writing Complete Ionic and Net Ionic Equations Write a complete ionic and net ionic equation for the following reaction. Solution: Complete ionic equation: Net ionic equation: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

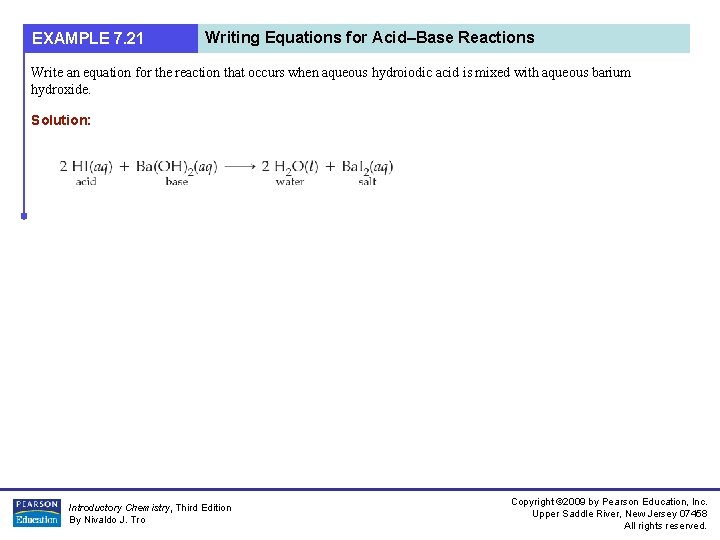
EXAMPLE 7. 21 Writing Equations for Acid–Base Reactions Write an equation for the reaction that occurs when aqueous hydroiodic acid is mixed with aqueous barium hydroxide. Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

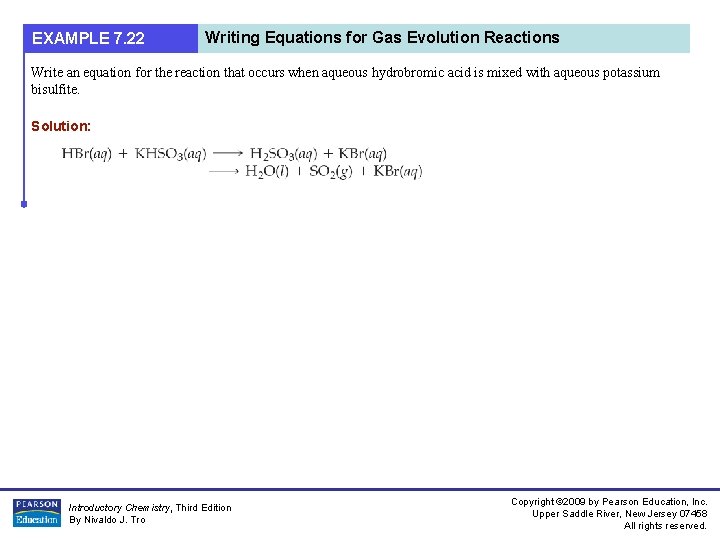
EXAMPLE 7. 22 Writing Equations for Gas Evolution Reactions Write an equation for the reaction that occurs when aqueous hydrobromic acid is mixed with aqueous potassium bisulfite. Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

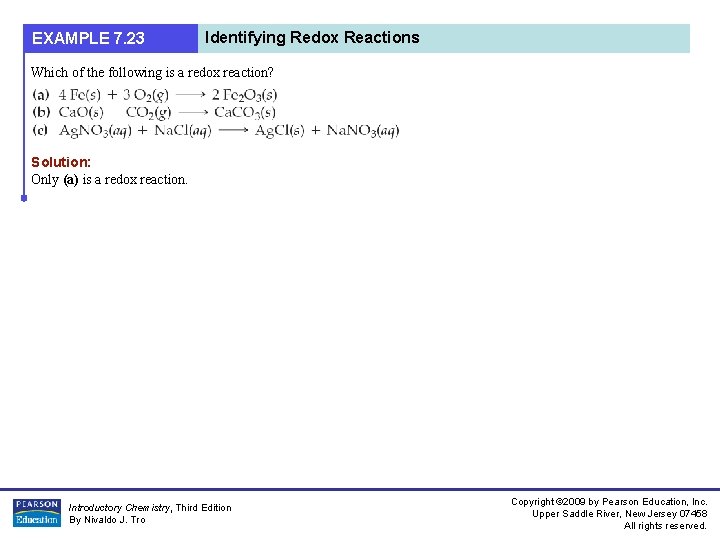
EXAMPLE 7. 23 Identifying Redox Reactions Which of the following is a redox reaction? Solution: Only (a) is a redox reaction. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

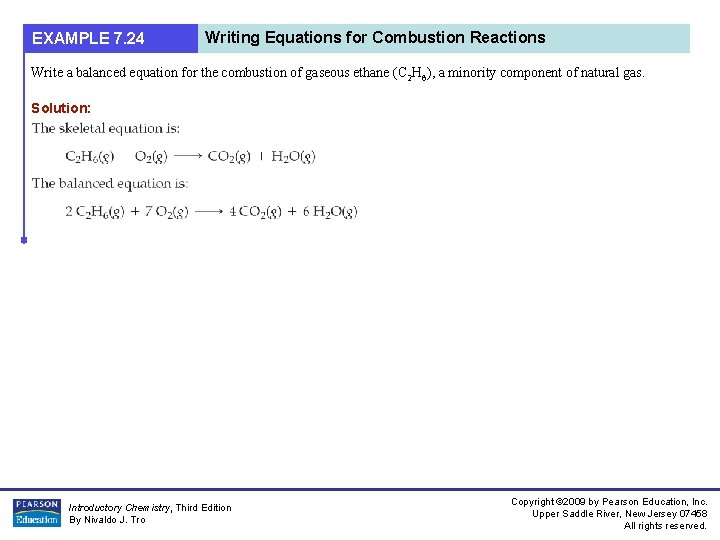
EXAMPLE 7. 24 Writing Equations for Combustion Reactions Write a balanced equation for the combustion of gaseous ethane (C 2 H 6), a minority component of natural gas. Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

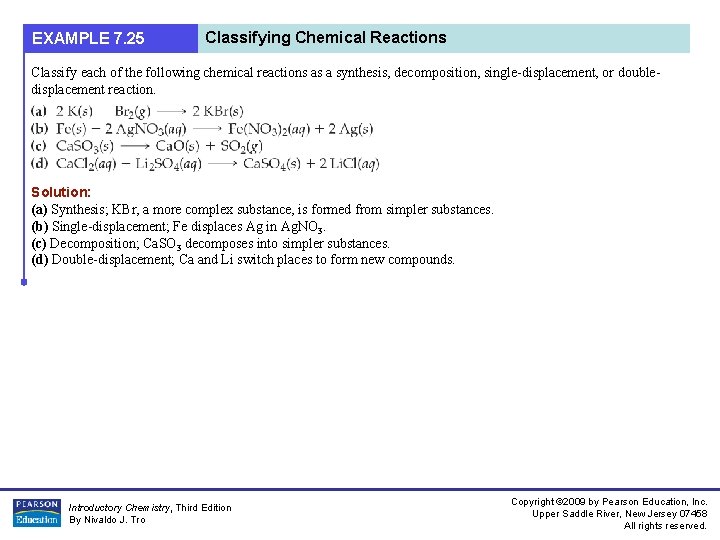
EXAMPLE 7. 25 Classifying Chemical Reactions Classify each of the following chemical reactions as a synthesis, decomposition, single-displacement, or doubledisplacement reaction. Solution: (a) Synthesis; KBr, a more complex substance, is formed from simpler substances. (b) Single-displacement; Fe displaces Ag in Ag. NO 3. (c) Decomposition; Ca. SO 3 decomposes into simpler substances. (d) Double-displacement; Ca and Li switch places to form new compounds. Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.