EXAMPLE 13 1 Calculating Mass Percent Calculate the

- Slides: 25

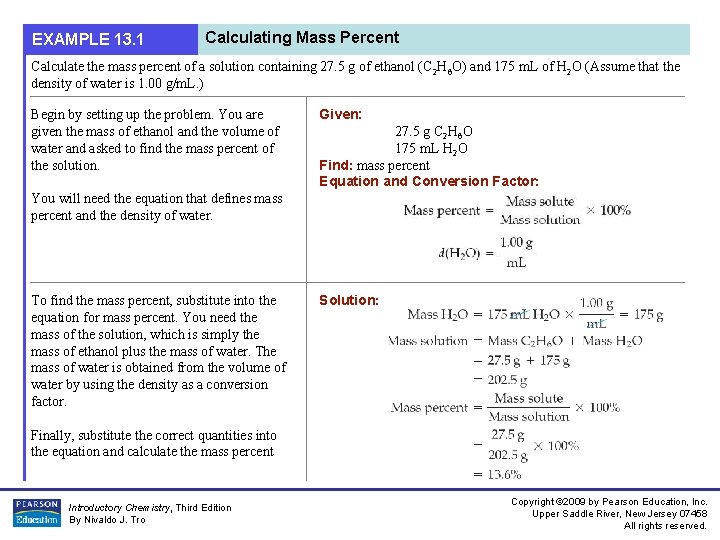
EXAMPLE 13. 1 Calculating Mass Percent Calculate the mass percent of a solution containing 27. 5 g of ethanol (C 2 H 6 O) and 175 m. L of H 2 O (Assume that the density of water is 1. 00 g/m. L. ) Begin by setting up the problem. You are given the mass of ethanol and the volume of water and asked to find the mass percent of the solution. Given: 27. 5 g C 2 H 6 O 175 m. L H 2 O Find: mass percent Equation and Conversion Factor: You will need the equation that defines mass percent and the density of water. To find the mass percent, substitute into the equation for mass percent. You need the mass of the solution, which is simply the mass of ethanol plus the mass of water. The mass of water is obtained from the volume of water by using the density as a conversion factor. Solution: Finally, substitute the correct quantities into the equation and calculate the mass percent Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

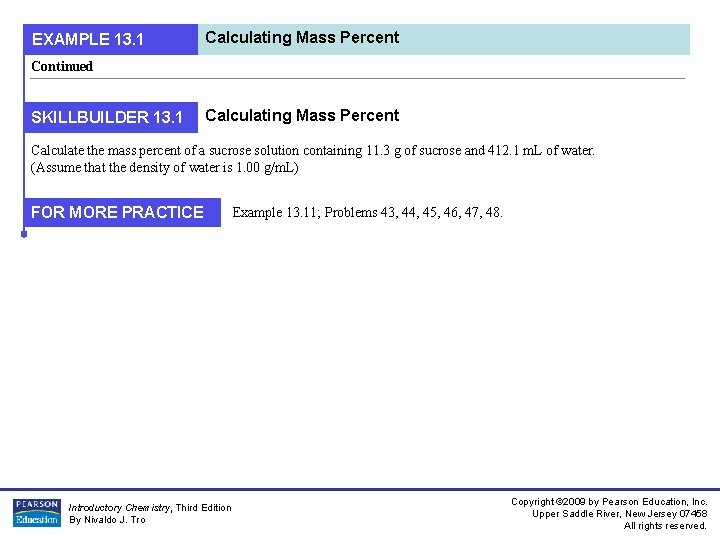
EXAMPLE 13. 1 Calculating Mass Percent Continued SKILLBUILDER 13. 1 Calculating Mass Percent Calculate the mass percent of a sucrose solution containing 11. 3 g of sucrose and 412. 1 m. L of water. (Assume that the density of water is 1. 00 g/m. L) FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 11; Problems 43, 44, 45, 46, 47, 48. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

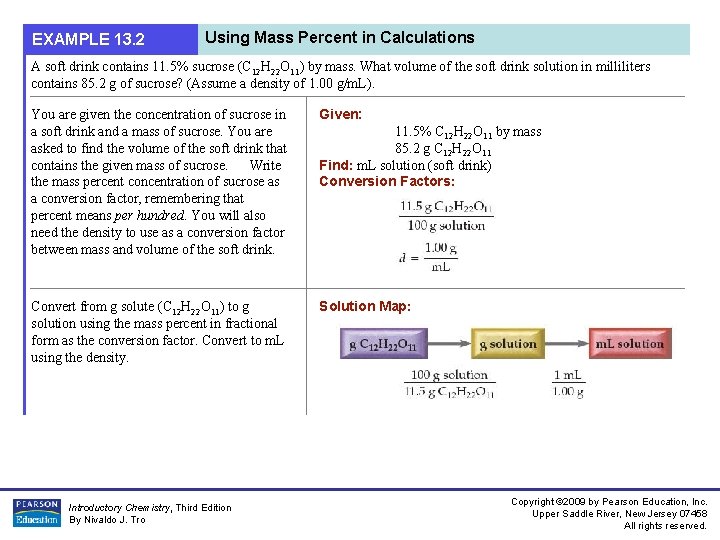
EXAMPLE 13. 2 Using Mass Percent in Calculations A soft drink contains 11. 5% sucrose (C 12 H 22 O 11) by mass. What volume of the soft drink solution in milliliters contains 85. 2 g of sucrose? (Assume a density of 1. 00 g/m. L). You are given the concentration of sucrose in a soft drink and a mass of sucrose. You are asked to find the volume of the soft drink that contains the given mass of sucrose. Write the mass percent concentration of sucrose as a conversion factor, remembering that percent means per hundred. You will also need the density to use as a conversion factor between mass and volume of the soft drink. Given: Convert from g solute (C 12 H 22 O 11) to g solution using the mass percent in fractional form as the conversion factor. Convert to m. L using the density. Solution Map: Introductory Chemistry, Third Edition By Nivaldo J. Tro 11. 5% C 12 H 22 O 11 by mass 85. 2 g C 12 H 22 O 11 Find: m. L solution (soft drink) Conversion Factors: Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

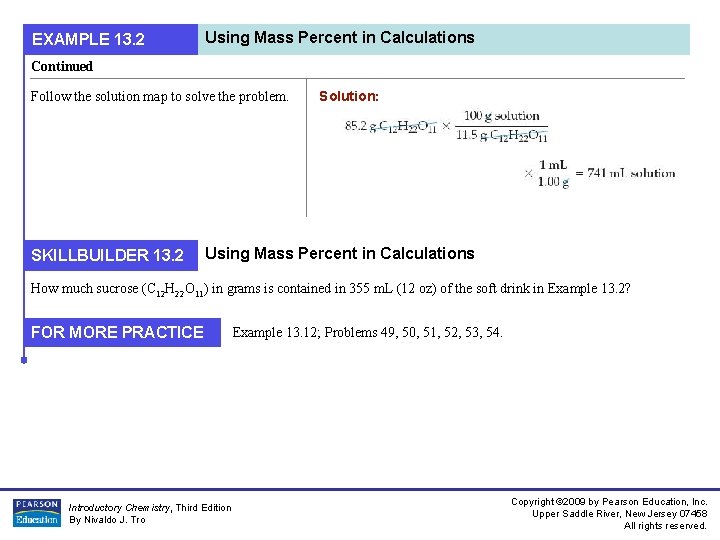
EXAMPLE 13. 2 Using Mass Percent in Calculations Continued Follow the solution map to solve the problem. SKILLBUILDER 13. 2 Solution: Using Mass Percent in Calculations How much sucrose (C 12 H 22 O 11) in grams is contained in 355 m. L (12 oz) of the soft drink in Example 13. 2? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 12; Problems 49, 50, 51, 52, 53, 54. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

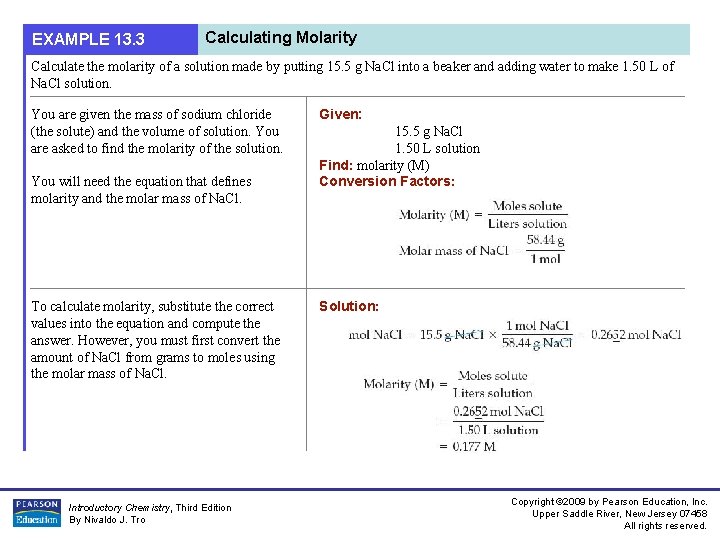
EXAMPLE 13. 3 Calculating Molarity Calculate the molarity of a solution made by putting 15. 5 g Na. Cl into a beaker and adding water to make 1. 50 L of Na. Cl solution. You are given the mass of sodium chloride (the solute) and the volume of solution. You are asked to find the molarity of the solution. You will need the equation that defines molarity and the molar mass of Na. Cl. To calculate molarity, substitute the correct values into the equation and compute the answer. However, you must first convert the amount of Na. Cl from grams to moles using the molar mass of Na. Cl. Introductory Chemistry, Third Edition By Nivaldo J. Tro Given: 15. 5 g Na. Cl 1. 50 L solution Find: molarity (M) Conversion Factors: Solution: Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 13. 3 Calculating Molarity Continued SKILLBUILDER 13. 3 Calculating Molarity Calculate the molarity of a solution made by putting 55. 8 g of Na. NO 3 into a beaker and diluting to 2. 50 L. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 13; Problems 61, 62, 63, 64, 65, 66. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

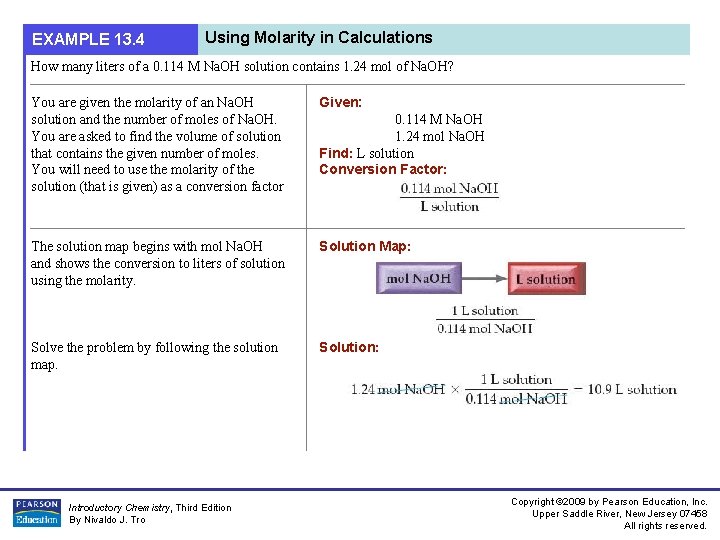
EXAMPLE 13. 4 Using Molarity in Calculations How many liters of a 0. 114 M Na. OH solution contains 1. 24 mol of Na. OH? You are given the molarity of an Na. OH solution and the number of moles of Na. OH. You are asked to find the volume of solution that contains the given number of moles. You will need to use the molarity of the solution (that is given) as a conversion factor Given: The solution map begins with mol Na. OH and shows the conversion to liters of solution using the molarity. Solution Map: Solve the problem by following the solution map. Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro 0. 114 M Na. OH 1. 24 mol Na. OH Find: L solution Conversion Factor: Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 13. 4 Calculating Molarity Continued SKILLBUILDER 13. 4 Using Molarity in Calculations How much of a 0. 225 M KCl solution contains 55. 8 g of KCl? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 14; Problems 67, 68, 69, 70, 71, 72. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

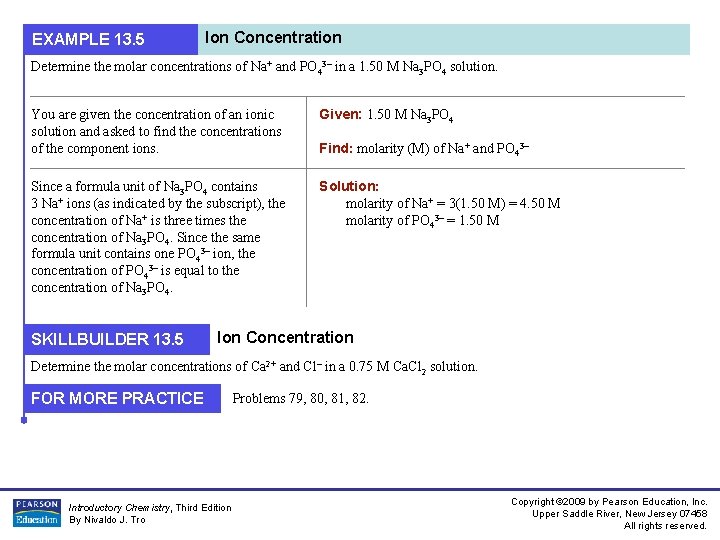
EXAMPLE 13. 5 Ion Concentration Determine the molar concentrations of Na+ and PO 43– in a 1. 50 M Na 3 PO 4 solution. You are given the concentration of an ionic solution and asked to find the concentrations of the component ions. Given: 1. 50 M Na 3 PO 4 Since a formula unit of Na 3 PO 4 contains 3 Na+ ions (as indicated by the subscript), the concentration of Na+ is three times the concentration of Na 3 PO 4. Since the same formula unit contains one PO 43– ion, the concentration of PO 43– is equal to the concentration of Na 3 PO 4. Solution: molarity of Na+ = 3(1. 50 M) = 4. 50 M molarity of PO 43– = 1. 50 M SKILLBUILDER 13. 5 Find: molarity (M) of Na+ and PO 43– Ion Concentration Determine the molar concentrations of Ca 2+ and Cl– in a 0. 75 M Ca. Cl 2 solution. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 79, 80, 81, 82. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 13. 6 Solution Dilution To what volume should you dilute 0. 100 L of a 15 M Na. OH solution to obtain a 1. 0 M Na. OH solution? You are given the initial volume and concentration of an Na. OH solution and a final concentration. You are asked to find the volume required to dilute the solution to the given final concentration. The necessary equation is the solution dilution equation given previously in this section. Solve the equation for V 2 the volume of the final solution, and substitute the required quantities to compute V 2. You would make the solution by diluting 0. 100 L of the stock solution to a total volume of 1. 5 L(V 2). The resulting solution will have a concentration of 1. 0 M. SKILLBUILDER 13. 6 Given: V 1 = 0. 100 L M 1 = 15 M M 2 = 1. 0 M Find: V 2 Equation: M 1 V 1 = M 2 V 2 Solution: Solution Dilution How much 6. 0 M Na. NO 3 solution should be used to make 0. 585 L of a 1. 2 M Na. NO 3 solution? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 15; Problems 83, 84, 85, 86, 87, 88, 89, 90. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

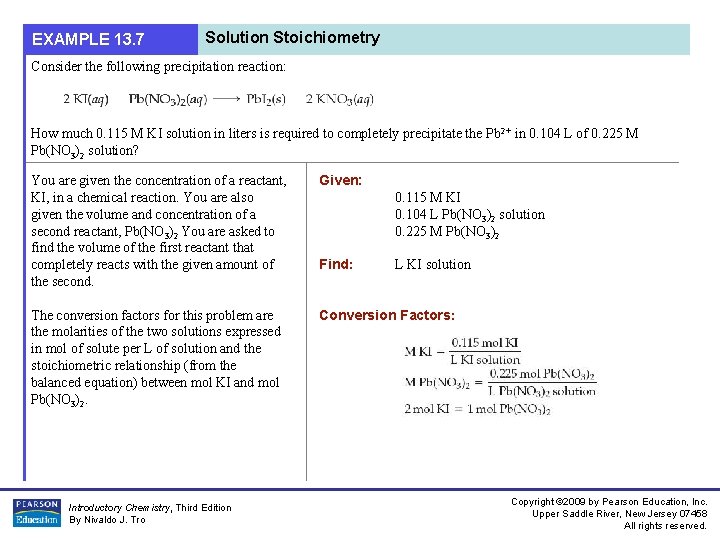
EXAMPLE 13. 7 Solution Stoichiometry Consider the following precipitation reaction: How much 0. 115 M KI solution in liters is required to completely precipitate the Pb 2+ in 0. 104 L of 0. 225 M Pb(NO 3)2 solution? You are given the concentration of a reactant, KI, in a chemical reaction. You are also given the volume and concentration of a second reactant, Pb(NO 3)2 You are asked to find the volume of the first reactant that completely reacts with the given amount of the second. Given: The conversion factors for this problem are the molarities of the two solutions expressed in mol of solute per L of solution and the stoichiometric relationship (from the balanced equation) between mol KI and mol Pb(NO 3)2. Conversion Factors: Introductory Chemistry, Third Edition By Nivaldo J. Tro 0. 115 M KI 0. 104 L Pb(NO 3)2 solution 0. 225 M Pb(NO 3)2 Find: L KI solution Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

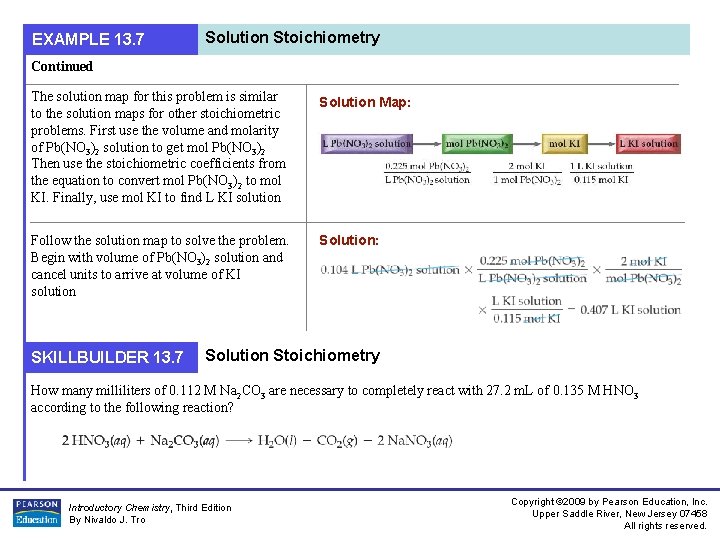
EXAMPLE 13. 7 Solution Stoichiometry Continued The solution map for this problem is similar to the solution maps for other stoichiometric problems. First use the volume and molarity of Pb(NO 3)2 solution to get mol Pb(NO 3)2 Then use the stoichiometric coefficients from the equation to convert mol Pb(NO 3)2 to mol KI. Finally, use mol KI to find L KI solution Solution Map: Follow the solution map to solve the problem. Begin with volume of Pb(NO 3)2 solution and cancel units to arrive at volume of KI solution Solution: SKILLBUILDER 13. 7 Solution Stoichiometry How many milliliters of 0. 112 M Na 2 CO 3 are necessary to completely react with 27. 2 m. L of 0. 135 M HNO 3 according to the following reaction? Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

EXAMPLE 13. 7 Solution Stoichiometry Continued SKILLBUILDER PLUS A 25. 0 -m. L sample of HNO 3 solution requires 35. 7 m. L of 0. 108 M Na 2 CO 3 to completely react with all of the HNO 3 in the solution. What was the concentration of the HNO 3 solution? FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 16; Problems 91, 92, 93, 94. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

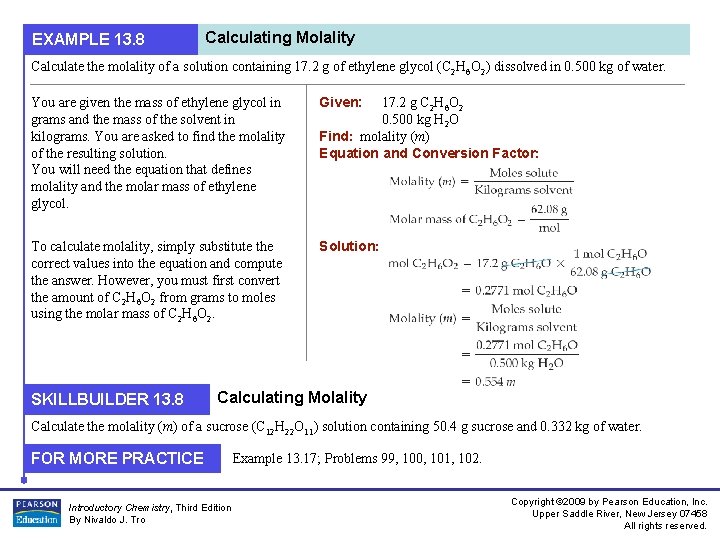
EXAMPLE 13. 8 Calculating Molality Calculate the molality of a solution containing 17. 2 g of ethylene glycol (C 2 H 6 O 2) dissolved in 0. 500 kg of water. You are given the mass of ethylene glycol in grams and the mass of the solvent in kilograms. You are asked to find the molality of the resulting solution. You will need the equation that defines molality and the molar mass of ethylene glycol. Given: To calculate molality, simply substitute the correct values into the equation and compute the answer. However, you must first convert the amount of C 2 H 6 O 2 from grams to moles using the molar mass of C 2 H 6 O 2. Solution: SKILLBUILDER 13. 8 17. 2 g C 2 H 6 O 2 0. 500 kg H 2 O Find: molality (m) Equation and Conversion Factor: Calculating Molality Calculate the molality (m) of a sucrose (C 12 H 22 O 11) solution containing 50. 4 g sucrose and 0. 332 kg of water. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 17; Problems 99, 100, 101, 102. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

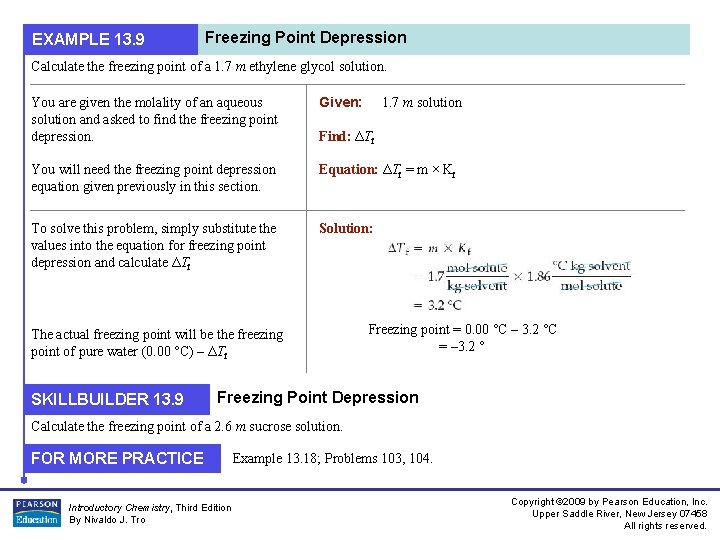
EXAMPLE 13. 9 Freezing Point Depression Calculate the freezing point of a 1. 7 m ethylene glycol solution. You are given the molality of an aqueous solution and asked to find the freezing point depression. Given: You will need the freezing point depression equation given previously in this section. Equation: ΔTf = m × Kf To solve this problem, simply substitute the values into the equation for freezing point depression and calculate ΔTf Solution: Find: ΔTf The actual freezing point will be the freezing point of pure water (0. 00 °C) – ΔTf SKILLBUILDER 13. 9 1. 7 m solution Freezing point = 0. 00 °C – 3. 2 °C = – 3. 2 ° Freezing Point Depression Calculate the freezing point of a 2. 6 m sucrose solution. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Example 13. 18; Problems 103, 104. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

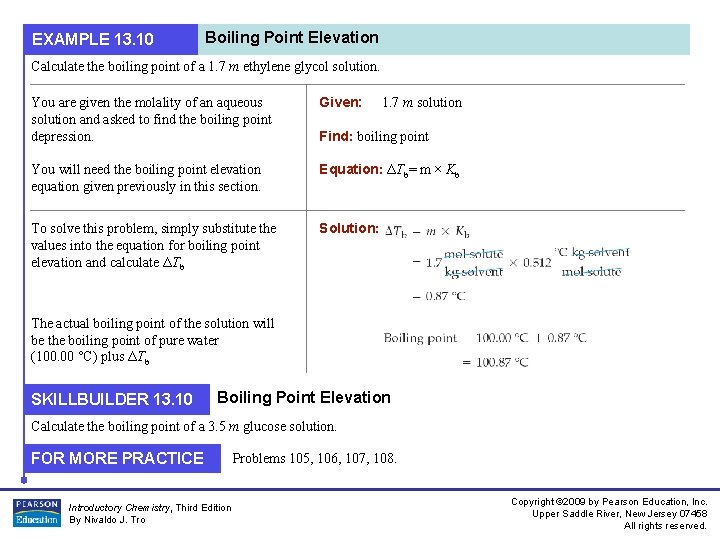
EXAMPLE 13. 10 Boiling Point Elevation Calculate the boiling point of a 1. 7 m ethylene glycol solution. You are given the molality of an aqueous solution and asked to find the boiling point depression. Given: You will need the boiling point elevation equation given previously in this section. Equation: ΔTb= m × Kb To solve this problem, simply substitute the values into the equation for boiling point elevation and calculate ΔTb Solution: 1. 7 m solution Find: boiling point The actual boiling point of the solution will be the boiling point of pure water (100. 00 °C) plus ΔTb SKILLBUILDER 13. 10 Boiling Point Elevation Calculate the boiling point of a 3. 5 m glucose solution. FOR MORE PRACTICE Introductory Chemistry, Third Edition By Nivaldo J. Tro Problems 105, 106, 107, 108. Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

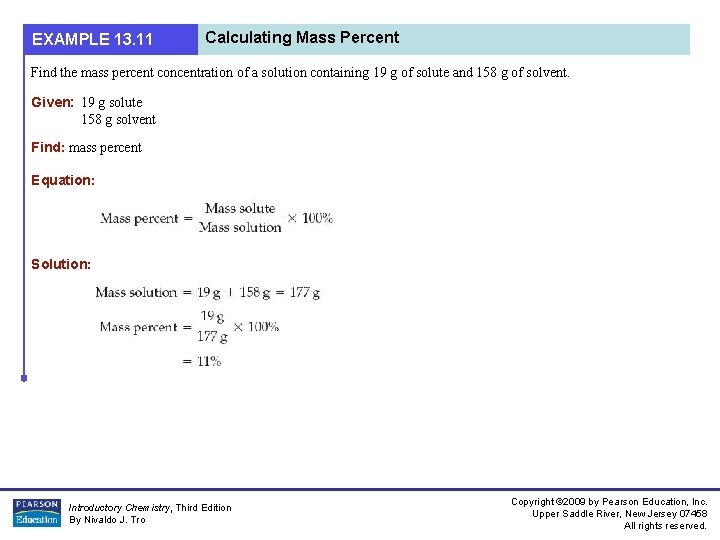
EXAMPLE 13. 11 Calculating Mass Percent Find the mass percent concentration of a solution containing 19 g of solute and 158 g of solvent. Given: 19 g solute 158 g solvent Find: mass percent Equation: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

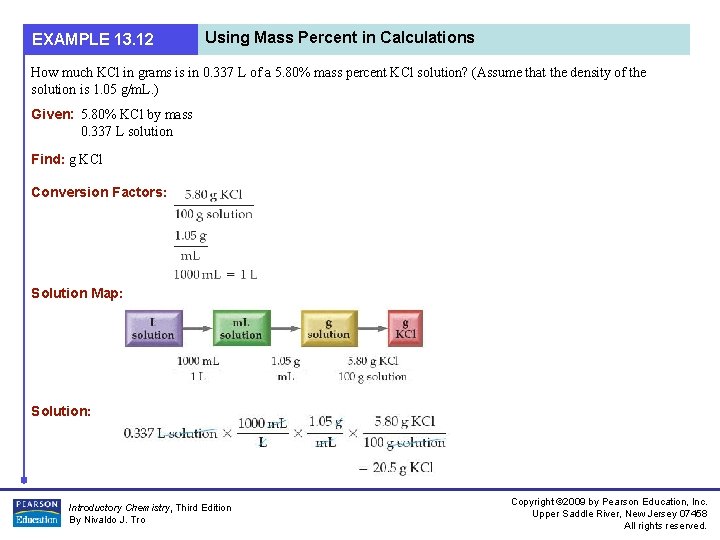
EXAMPLE 13. 12 Using Mass Percent in Calculations How much KCl in grams is in 0. 337 L of a 5. 80% mass percent KCl solution? (Assume that the density of the solution is 1. 05 g/m. L. ) Given: 5. 80% KCl by mass 0. 337 L solution Find: g KCl Conversion Factors: Solution Map: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

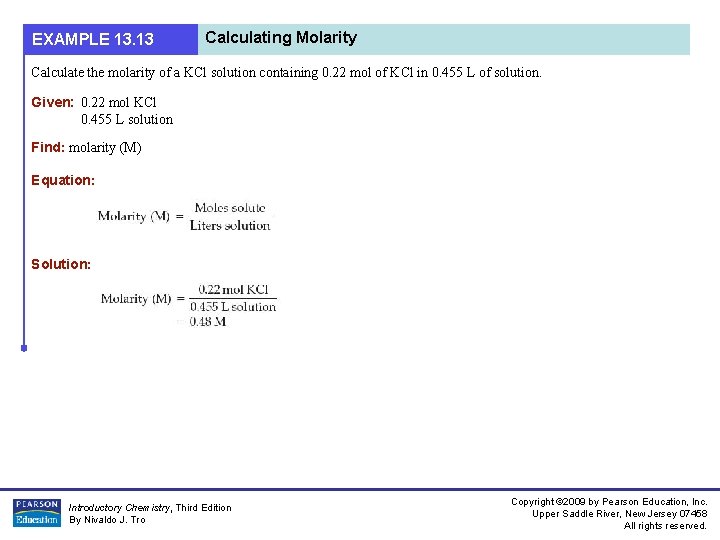
EXAMPLE 13. 13 Calculating Molarity Calculate the molarity of a KCl solution containing 0. 22 mol of KCl in 0. 455 L of solution. Given: 0. 22 mol KCl 0. 455 L solution Find: molarity (M) Equation: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

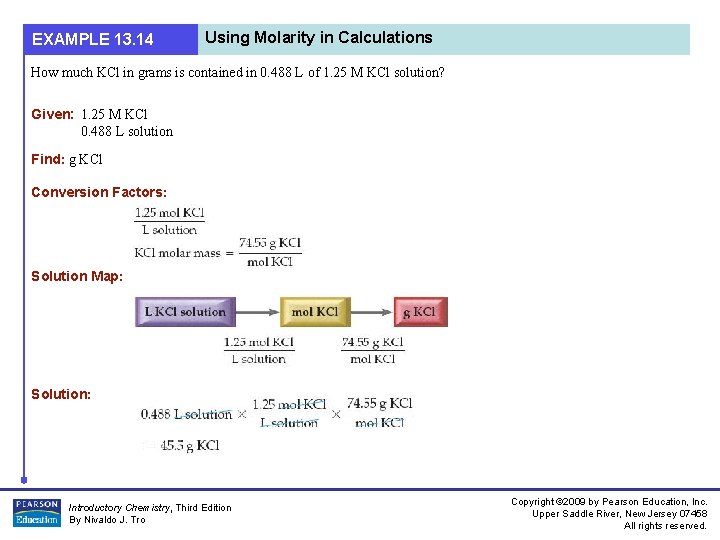
EXAMPLE 13. 14 Using Molarity in Calculations How much KCl in grams is contained in 0. 488 L of 1. 25 M KCl solution? Given: 1. 25 M KCl 0. 488 L solution Find: g KCl Conversion Factors: Solution Map: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

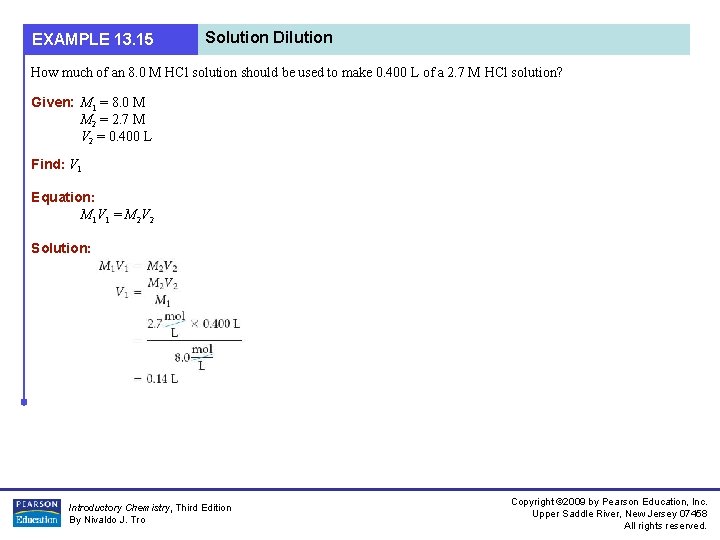
EXAMPLE 13. 15 Solution Dilution How much of an 8. 0 M HCl solution should be used to make 0. 400 L of a 2. 7 M HCl solution? Given: M 1 = 8. 0 M M 2 = 2. 7 M V 2 = 0. 400 L Find: V 1 Equation: M 1 V 1 = M 2 V 2 Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

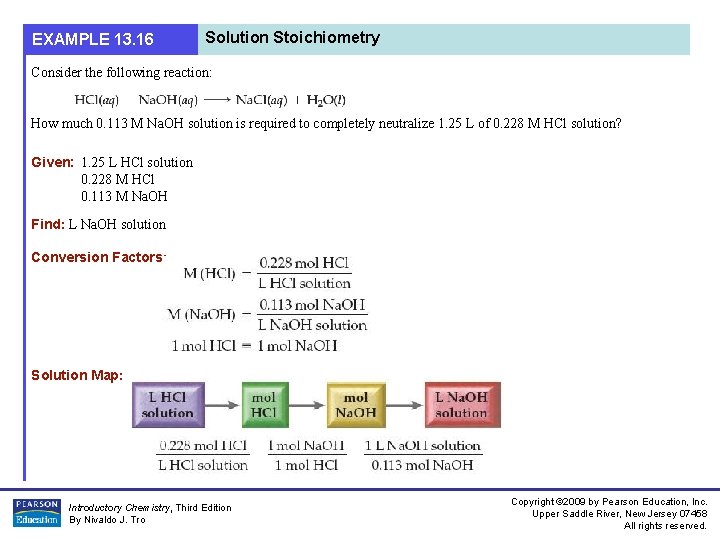
EXAMPLE 13. 16 Solution Stoichiometry Consider the following reaction: How much 0. 113 M Na. OH solution is required to completely neutralize 1. 25 L of 0. 228 M HCl solution? Given: 1. 25 L HCl solution 0. 228 M HCl 0. 113 M Na. OH Find: L Na. OH solution Conversion Factors: Solution Map: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

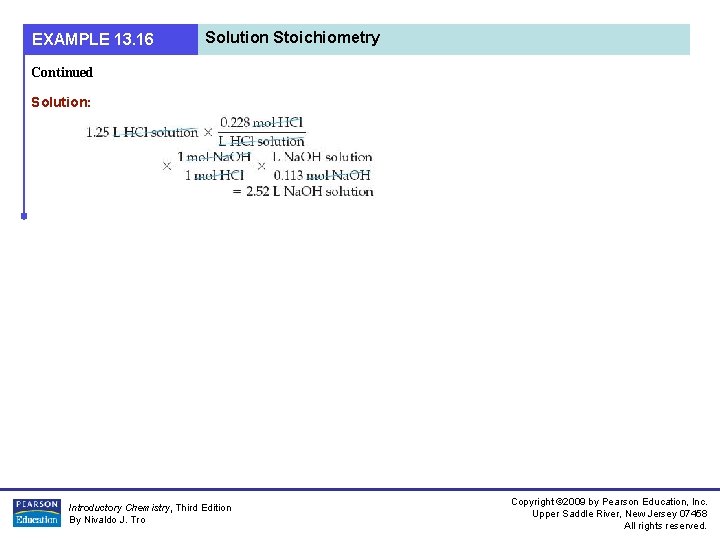
EXAMPLE 13. 16 Solution Stoichiometry Continued Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

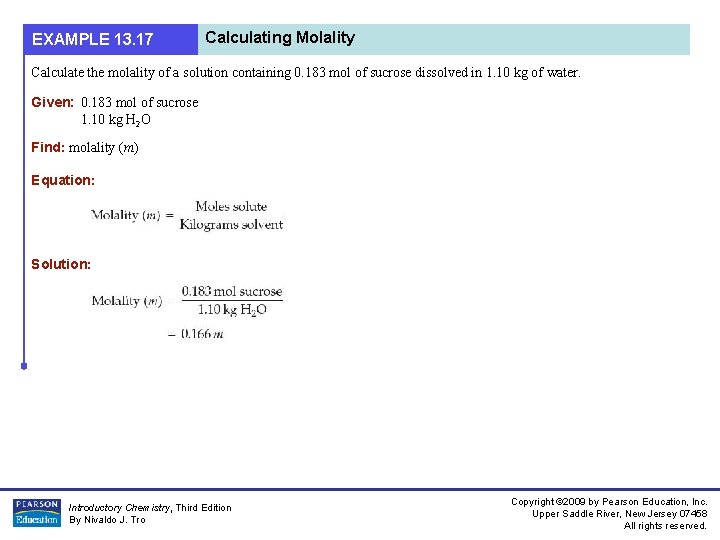
EXAMPLE 13. 17 Calculating Molality Calculate the molality of a solution containing 0. 183 mol of sucrose dissolved in 1. 10 kg of water. Given: 0. 183 mol of sucrose 1. 10 kg H 2 O Find: molality (m) Equation: Solution: Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.

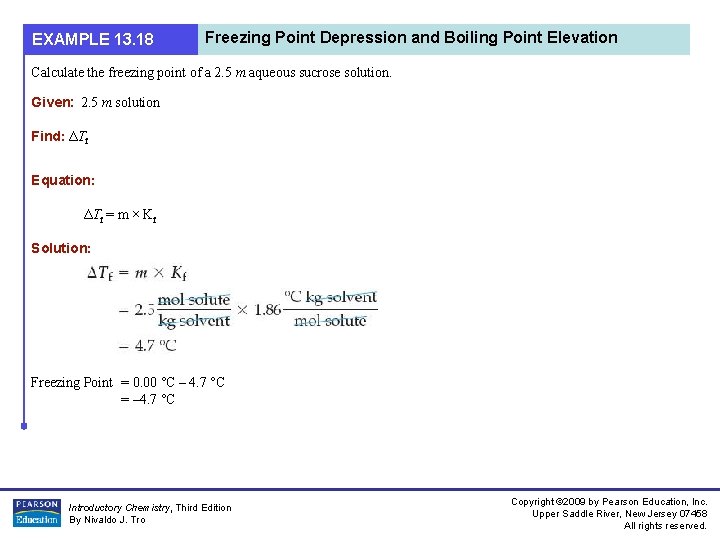
EXAMPLE 13. 18 Freezing Point Depression and Boiling Point Elevation Calculate the freezing point of a 2. 5 m aqueous sucrose solution. Given: 2. 5 m solution Find: ΔTf Equation: ΔTf = m × Kf Solution: Freezing Point = 0. 00 °C – 4. 7 °C = – 4. 7 °C Introductory Chemistry, Third Edition By Nivaldo J. Tro Copyright © 2009 by Pearson Education, Inc. Upper Saddle River, New Jersey 07458 All rights reserved.