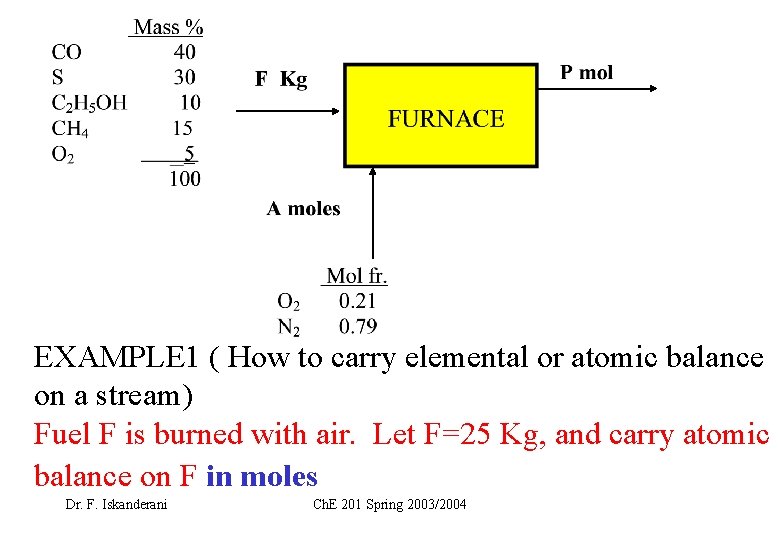
EXAMPLE 1 How to carry elemental or atomic




- Slides: 21

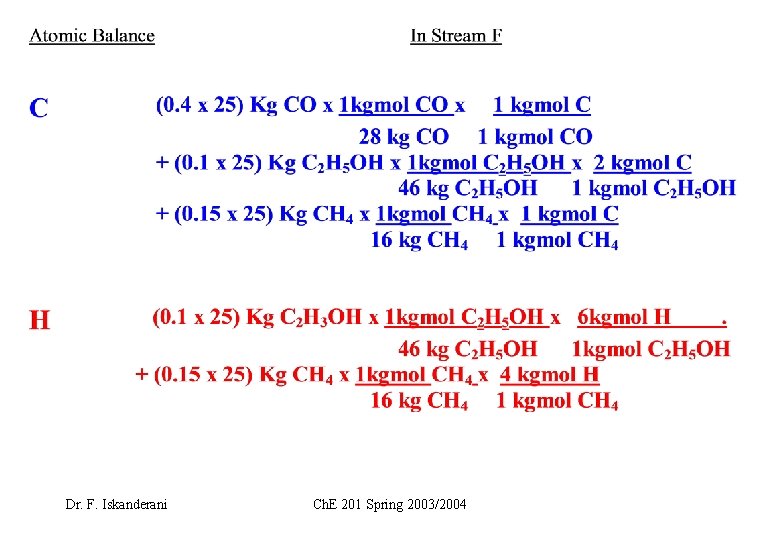
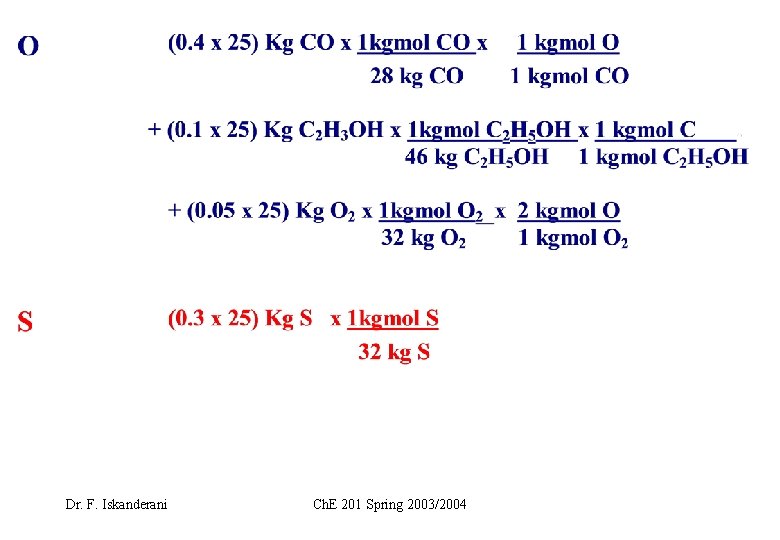
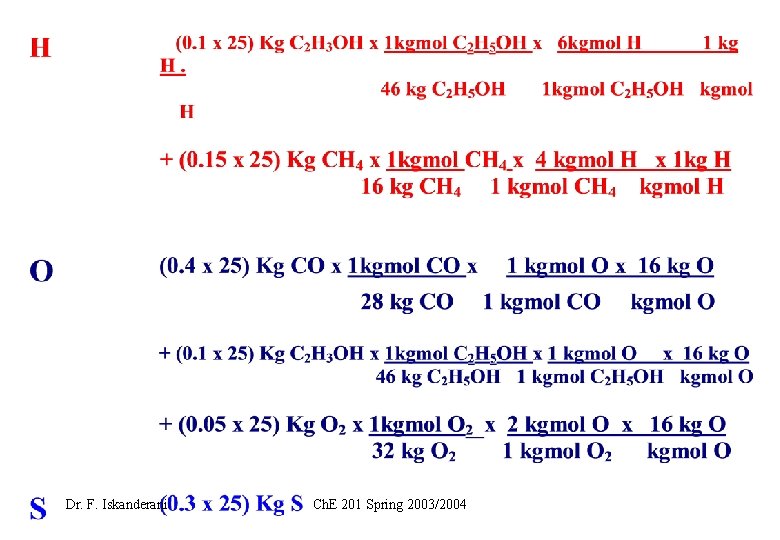
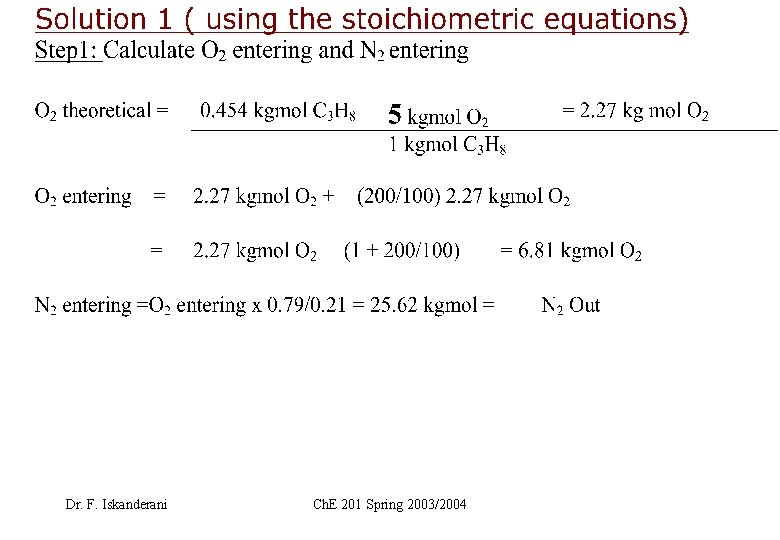
EXAMPLE 1 ( How to carry elemental or atomic balance on a stream) Fuel F is burned with air. Let F=25 Kg, and carry atomic balance on F in moles Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

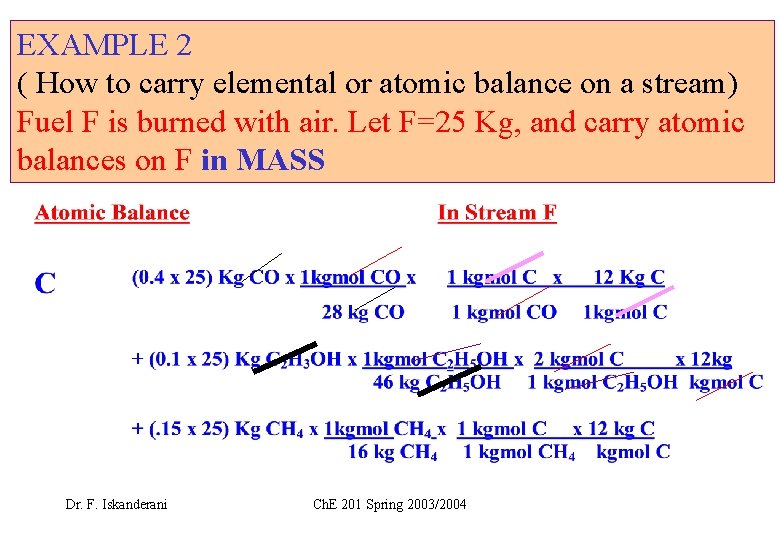
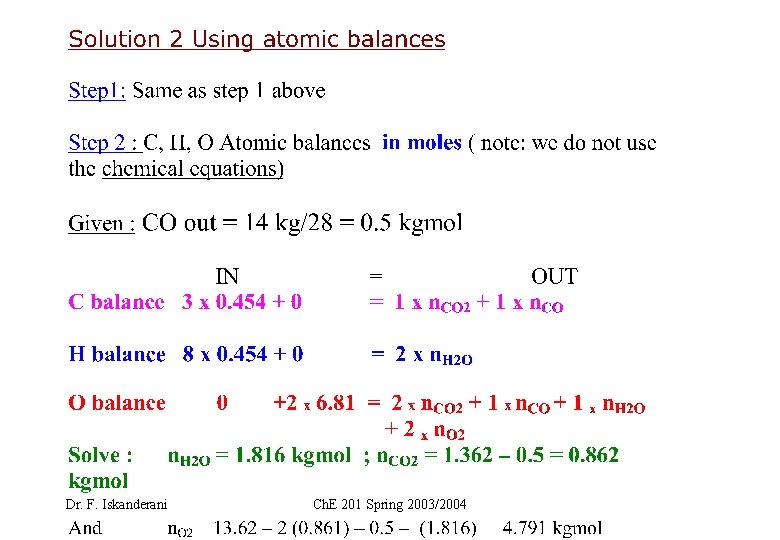
EXAMPLE 2 ( How to carry elemental or atomic balance on a stream) Fuel F is burned with air. Let F=25 Kg, and carry atomic balances on F in MASS Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

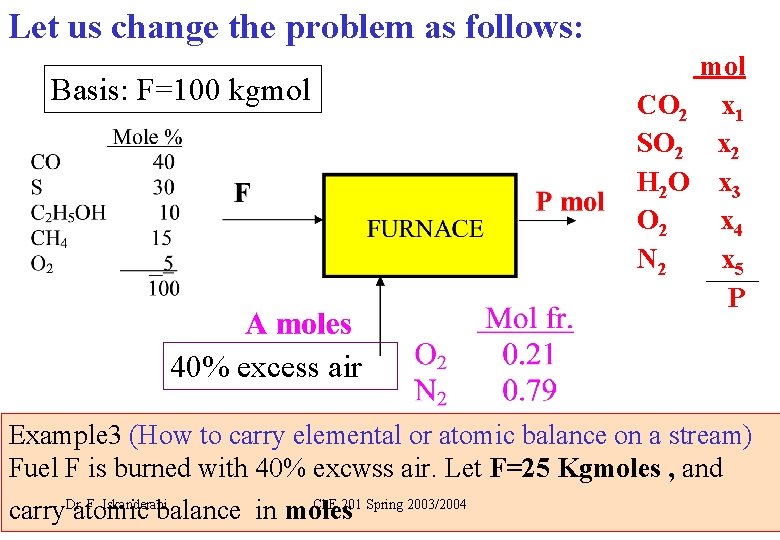
Let us change the problem as follows: Basis: F=100 kgmol CO 2 x 1 SO 2 x 2 H 2 O x 3 O 2 x 4 N 2 x 5 P 40% excess air Example 3 (How to carry elemental or atomic balance on a stream) Fuel F is burned with 40% excwss air. Let F=25 Kgmoles , and F. Iskanderani Ch. E 201 Spring 2003/2004 carry. Dr. atomic balance in moles

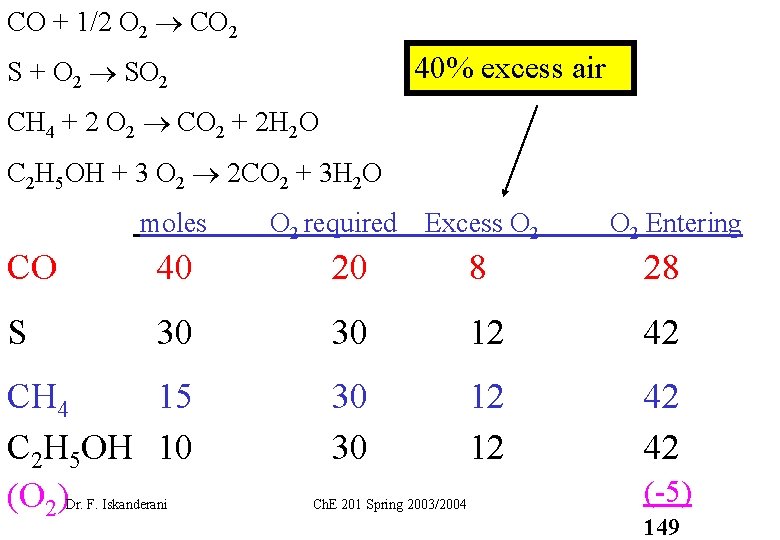
CO + 1/2 O 2 CO 2 40% excess air S + O 2 SO 2 CH 4 + 2 O 2 CO 2 + 2 H 2 O C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O moles O 2 required Excess O 2 Entering CO 40 20 8 28 S 30 30 12 42 CH 4 15 C 2 H 5 OH 10 (O 2) 30 30 12 12 42 42 Dr. F. Iskanderani Ch. E 201 Spring 2003/2004 (-5) 149

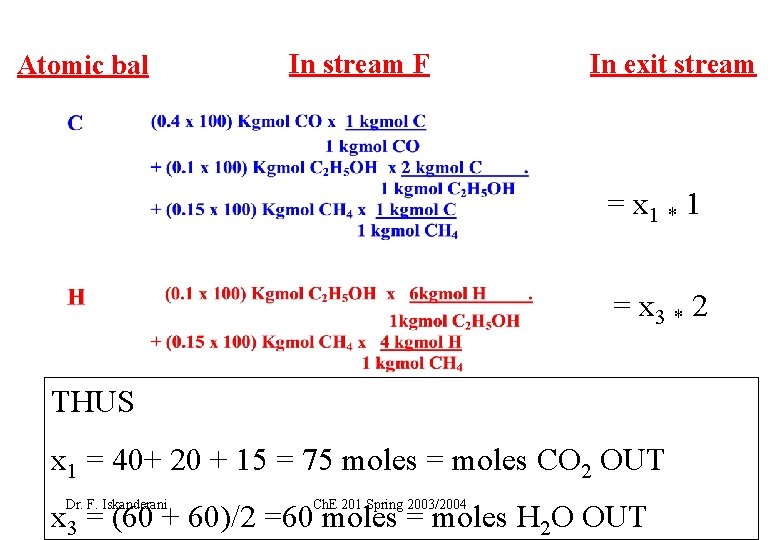
Atomic bal In stream F In exit stream = x 1 * 1 = x 3 * 2 THUS x 1 = 40+ 20 + 15 = 75 moles = moles CO 2 OUT Dr. F. Iskanderani Ch. E 201 Spring 2003/2004 x 3 = (60 + 60)/2 =60 moles = moles H 2 O OUT

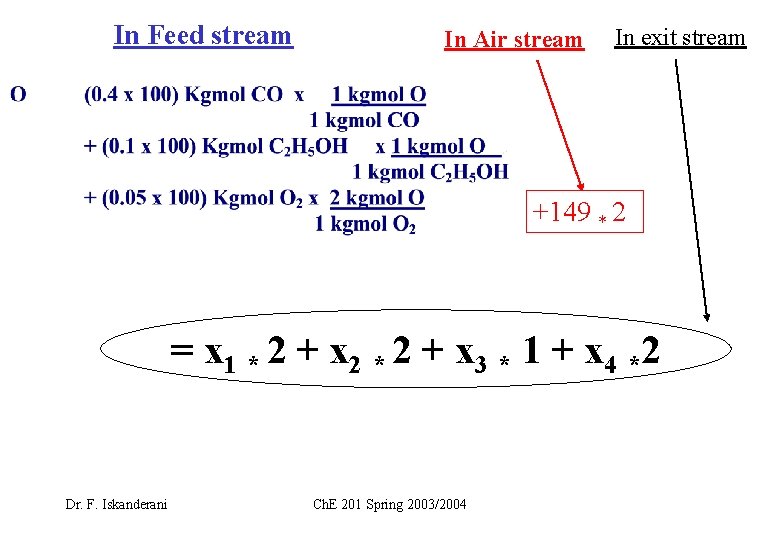
In Feed stream In Air stream In exit stream +149 * 2 = x 1 * 2 + x 2 * 2 + x 3 * 1 + x 4 *2 Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

S Balance mol S 0. 3 * 100 kgmol S = x 2 mol SO 2 *11 Mol SO 2 Thus x 2 = 30 moles = moles SO 2 OUT Now we can find x 4 Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

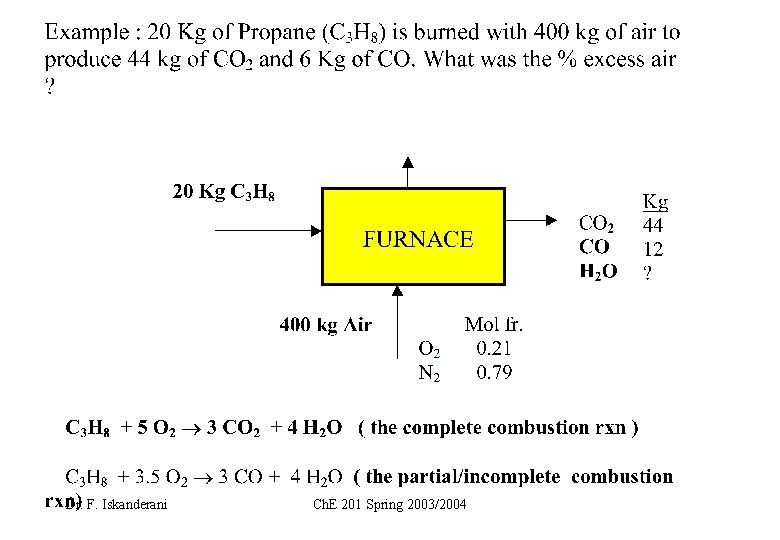
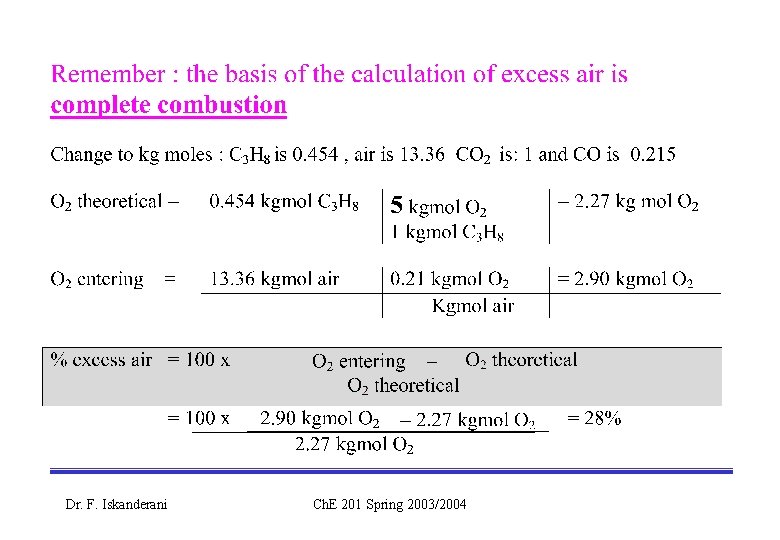
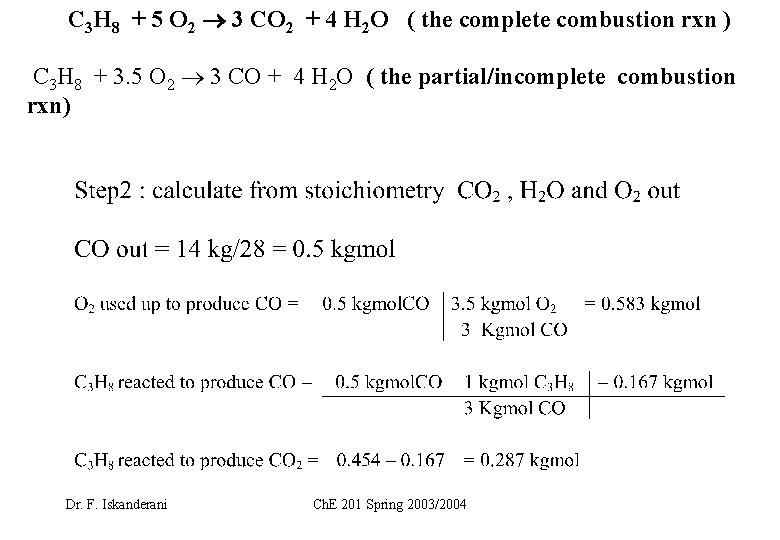
C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O ( the complete combustion rxn ) C 3 H 8 + 3. 5 O 2 3 CO + 4 H 2 O ( the partial/incomplete combustion rxn) Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

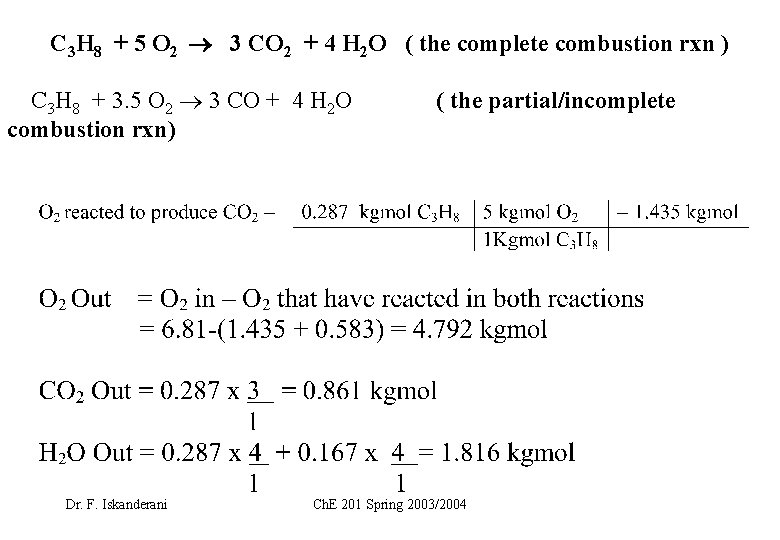
C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O ( the complete combustion rxn ) C 3 H 8 + 3. 5 O 2 3 CO + 4 H 2 O combustion rxn) Dr. F. Iskanderani ( the partial/incomplete Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004

Dr. F. Iskanderani Ch. E 201 Spring 2003/2004