EXAM 3 Review summary EXAM SCHEDULED FOR MONDAY
- Slides: 2

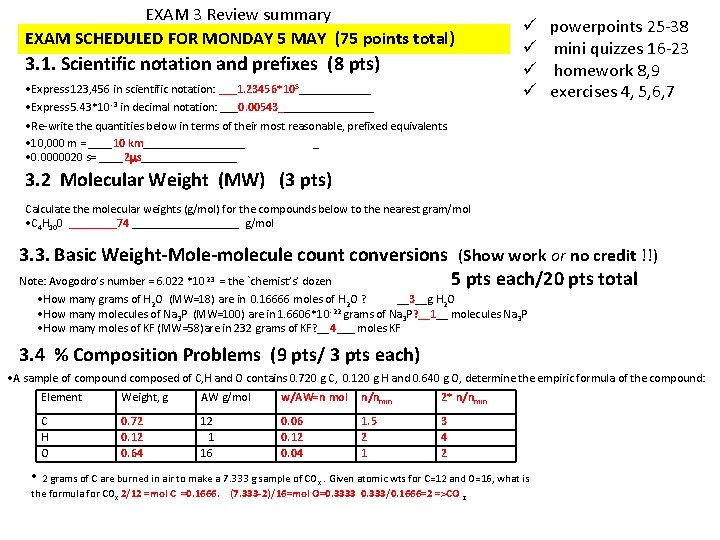
EXAM 3 Review summary EXAM SCHEDULED FOR MONDAY 5 MAY (75 points total) 3. 1. Scientific notation and prefixes (8 pts) • Express 123, 456 in scientific notation: ___1. 23456*10 5______ • Express 5. 43*10 -3 in decimal notation: ___0. 00543________ ü ü powerpoints 25 -38 mini quizzes 16 -23 homework 8, 9 exercises 4, 5, 6, 7 • Re-write the quantities below in terms of their most reasonable, prefixed equivalents. • 10, 000 m = ____10 km_________ _ • 0. 0000020 s= ____2 s________ 3. 2 Molecular Weight (MW) (3 pts) Calculate the molecular weights (g/mol) for the compounds below to the nearest gram/mol • C 4 H 100 ____74 _________ g/mol 3. 3. Basic Weight-Mole-molecule count conversions (Show work or no credit !!) Note: Avogodro’s number = 6. 022 *10 = the `chemist’s’ dozen 5 pts each/20 pts total 23 • How many grams of H 2 O (MW=18) are in 0. 16666 moles of H 2 O ? __3__g H 2 O -22 • How many molecules of Na 3 P (MW=100) are in 1. 6606*10 grams of Na 3 P? __1__ molecules Na 3 P • How many moles of KF (MW=58)are in 232 grams of KF? __4___ moles KF 3. 4 % Composition Problems (9 pts/ 3 pts each) • A sample of compound composed of C, H and O contains 0. 720 g C, 0. 120 g H and 0. 640 g O, determine the empiric formula of the compound: Element Weight, g AW g/mol w/AW=n mol n/nmin 2* n/nmin C H O 0. 72 0. 12 0. 64 12 1 16 0. 06 0. 12 0. 04 1. 5 2 1 3 4 2 • 2 grams of C are burned in air to make a 7. 333 g sample of CO . Given atomic wts for C=12 and O=16, what is the formula for COx 2/12 =mol C =0. 1666. (7. 333 -2)/16=mol O=0. 3333 0. 333/0. 1666=2 =>CO 2 x

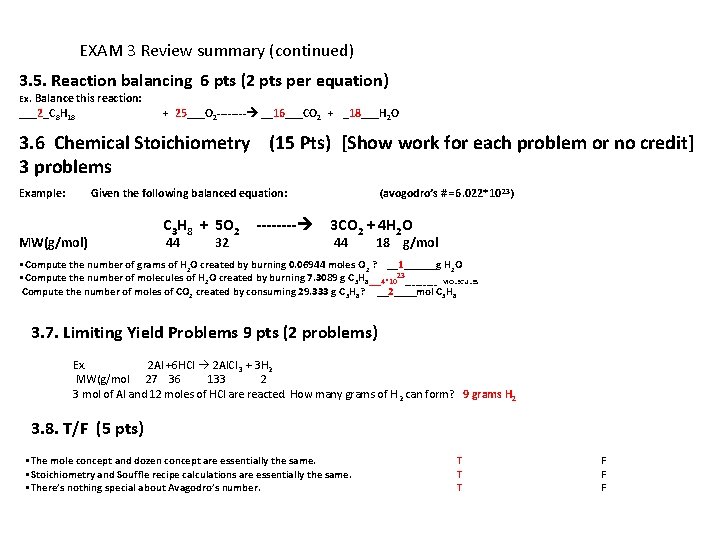
EXAM 3 Review summary (continued) 3. 5. Reaction balancing 6 pts (2 pts per equation) Ex. Balance this reaction: ___2_C 8 H 18 + 25___O 2 ---- __16___CO 2 + _18___H 2 O 3. 6 Chemical Stoichiometry (15 Pts) [Show work for each problem or no credit] 3 problems Example: Given the following balanced equation: MW(g/mol) C 3 H 8 + 5 O 2 44 32 ---- (avogodro’s # =6. 022*10 23) 3 CO 2 + 4 H 2 O 44 18 g/mol • Compute the number of grams of H 2 O created by burning 0. 06944 moles O 2 ? __1______g H 2 O • Compute the number of molecules of H 2 O created by burning 7. 3089 g C 3 H 8___4*1023_____ MOLECULES Compute the number of moles of CO 2 created by consuming 29. 333 g C 3 H 8? __2____mol C 3 H 8 3. 7. Limiting Yield Problems 9 pts (2 problems) Ex. 2 Al +6 HCl 2 Al. Cl 3 + 3 H 2 MW(g/mol 27 36 133 2 3 mol of Al and 12 moles of HCl are reacted. How many grams of H 2 can form? 9 grams H 2 3. 8. T/F (5 pts) • The mole concept and dozen concept are essentially the same. • Stoichiometry and Souffle recipe calculations are essentially the same. • There’s nothing special about Avagodro’s number. T T T F F F