Exam 2 Review Chemical Equilibria and Acids Bases

Exam 2 Review Chemical Equilibria and Acids & Bases Geoffrey Geberth 10/1/18

Chemical Equilibria 2
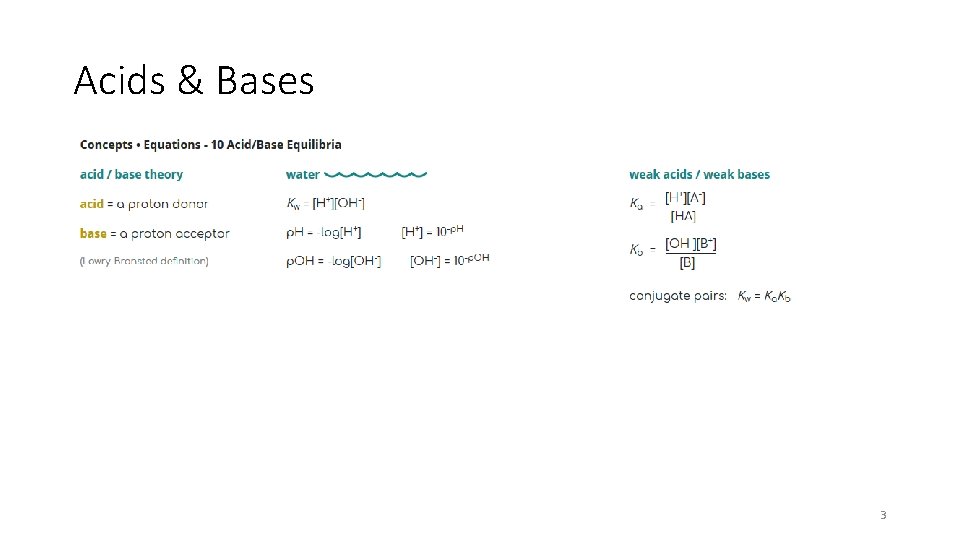
Acids & Bases 3

Activity (ai) • 4
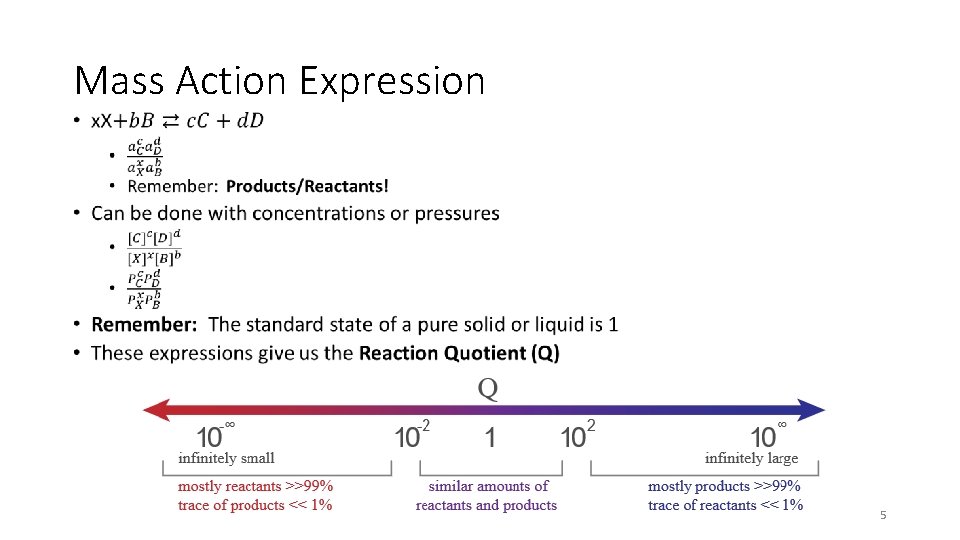
Mass Action Expression • 5

Equilibrium Constant • 6

Manipulating K • 7
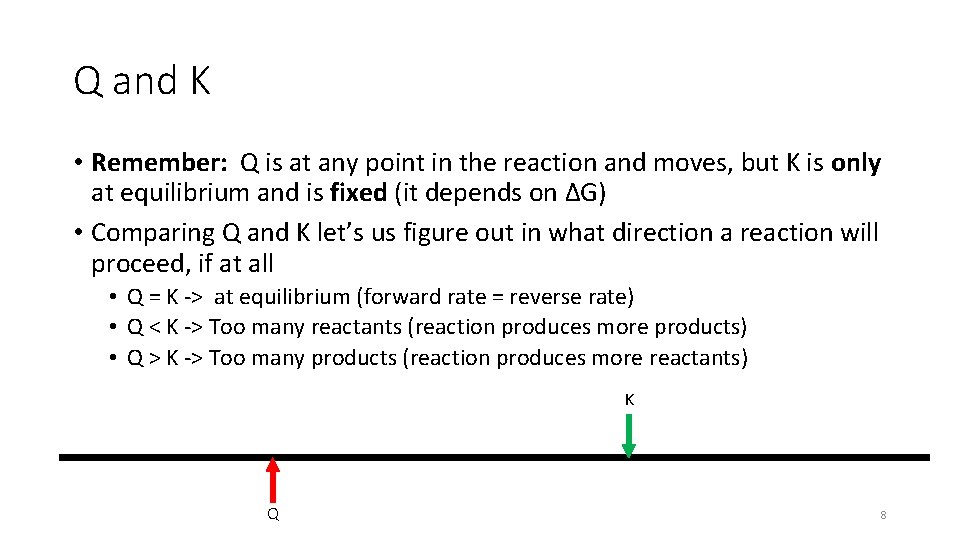
Q and K • Remember: Q is at any point in the reaction and moves, but K is only at equilibrium and is fixed (it depends on ΔG) • Comparing Q and K let’s us figure out in what direction a reaction will proceed, if at all • Q = K -> at equilibrium (forward rate = reverse rate) • Q < K -> Too many reactants (reaction produces more products) • Q > K -> Too many products (reaction produces more reactants) K Q 8
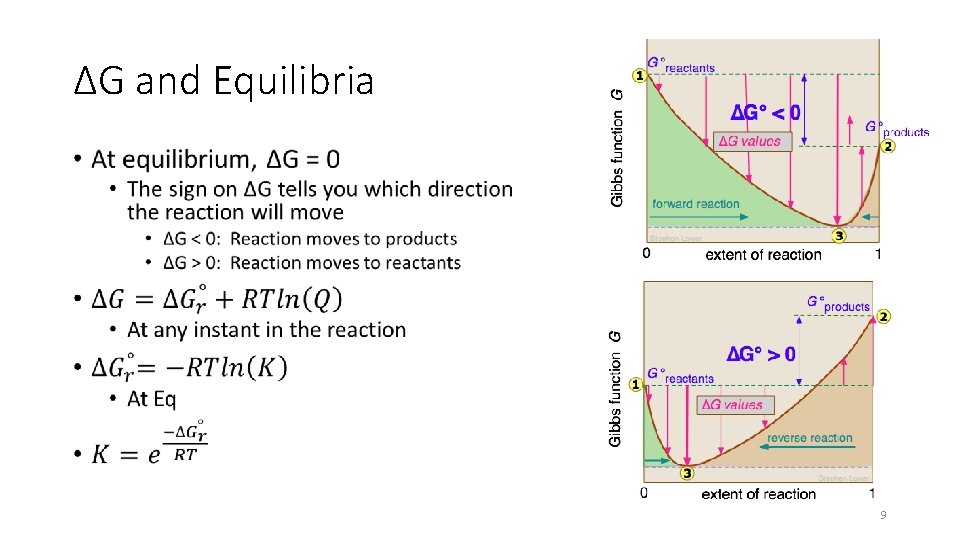
ΔG and Equilibria • 9

Let’s look at this equation more • 10

RICE tables • 11

Van’t Hoff Equation • 12

Definitions • 13
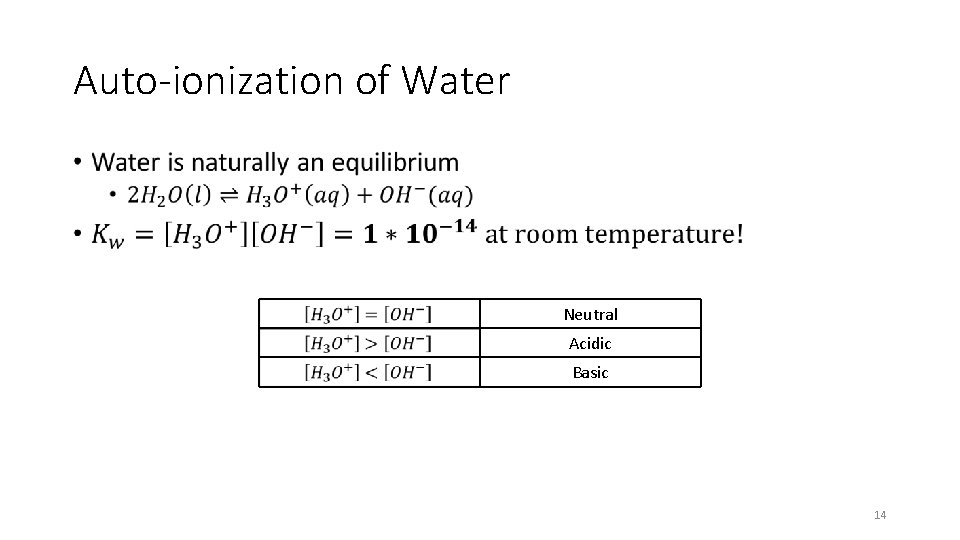
Auto-ionization of Water • Neutral Acidic Basic 14
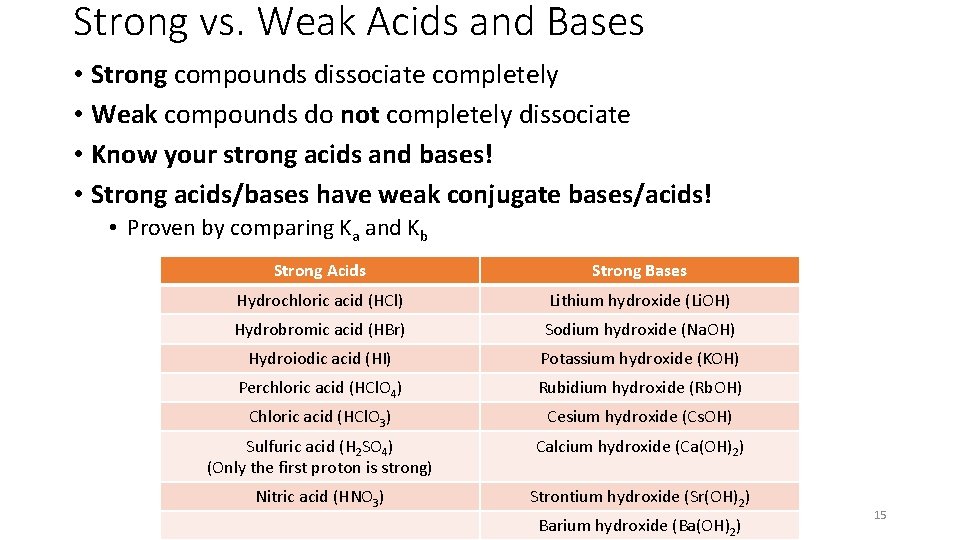
Strong vs. Weak Acids and Bases • Strong compounds dissociate completely • Weak compounds do not completely dissociate • Know your strong acids and bases! • Strong acids/bases have weak conjugate bases/acids! • Proven by comparing Ka and Kb Strong Acids Strong Bases Hydrochloric acid (HCl) Lithium hydroxide (Li. OH) Hydrobromic acid (HBr) Sodium hydroxide (Na. OH) Hydroiodic acid (HI) Potassium hydroxide (KOH) Perchloric acid (HCl. O 4) Rubidium hydroxide (Rb. OH) Chloric acid (HCl. O 3) Cesium hydroxide (Cs. OH) Sulfuric acid (H 2 SO 4) (Only the first proton is strong) Calcium hydroxide (Ca(OH)2) Nitric acid (HNO 3) Strontium hydroxide (Sr(OH)2) Barium hydroxide (Ba(OH)2) 15
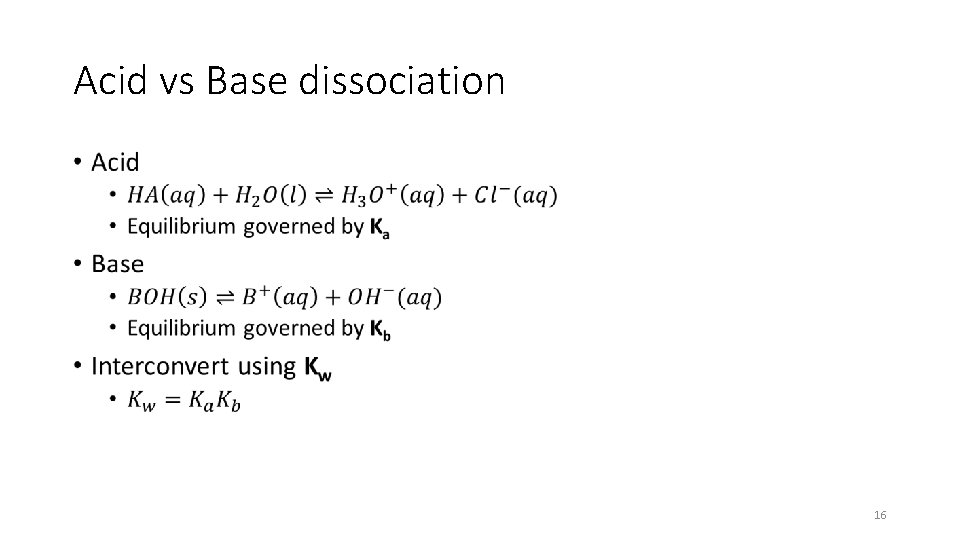
Acid vs Base dissociation • 16

Acid Base Equilibria • 17

Measures of Acidity and Basicity • 18

% ionization • 19

Let’s practice! • 20
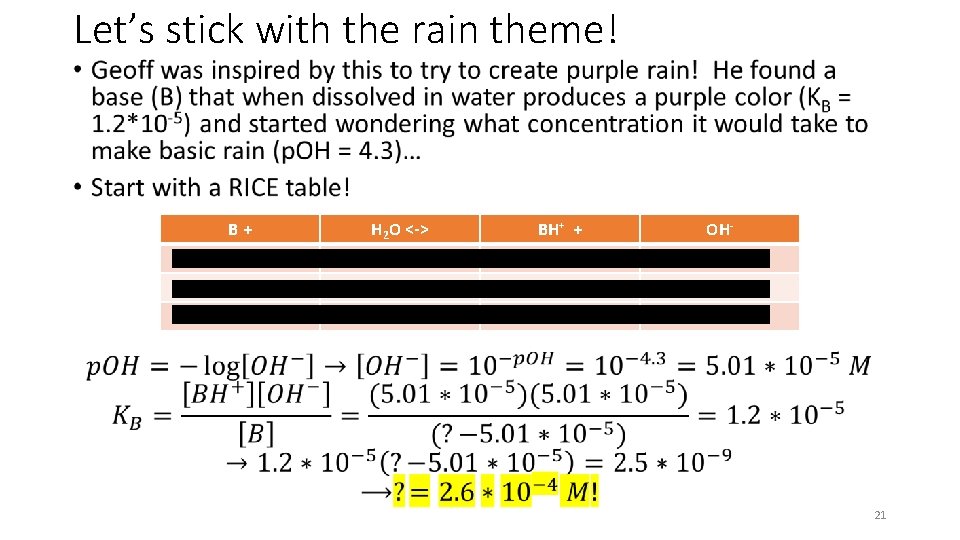
Let’s stick with the rain theme! • B+ H 2 O <-> BH+ + OH- ? - 0 0 -X - +X +X ? – 0. 014 M - 0. 014 M 21

Who’ll Stop the. RAINING Rain? ! IT’S MEN! • While preparing his purple rain solution, he looked outside and noticed something. The weather had changed again… (Hallelujah!) Image taken from the offical music video for It’s Raining Men by the Weather Girls. I do not own any rights to the song 22 I am citing under or video. academic fair use.

Time for a tough problem! • 23
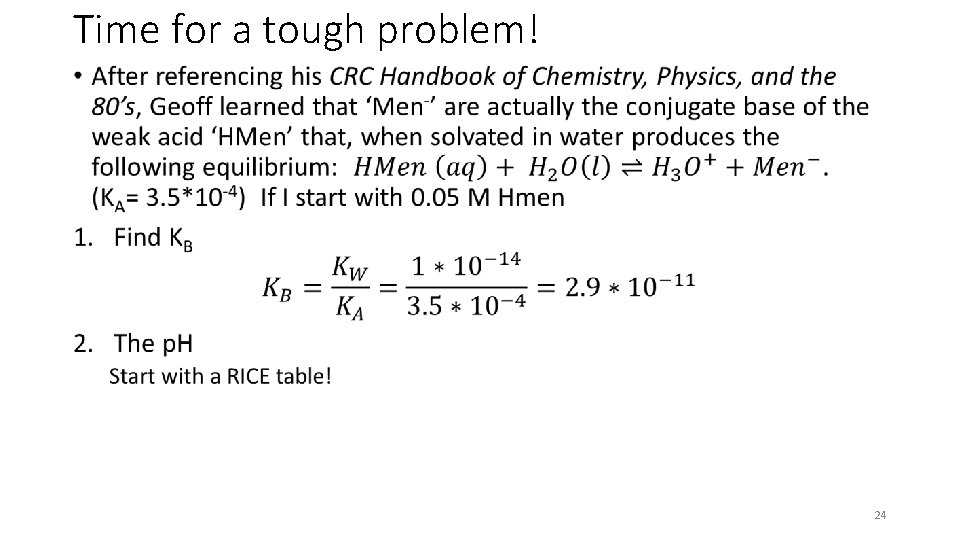
Time for a tough problem! • 24
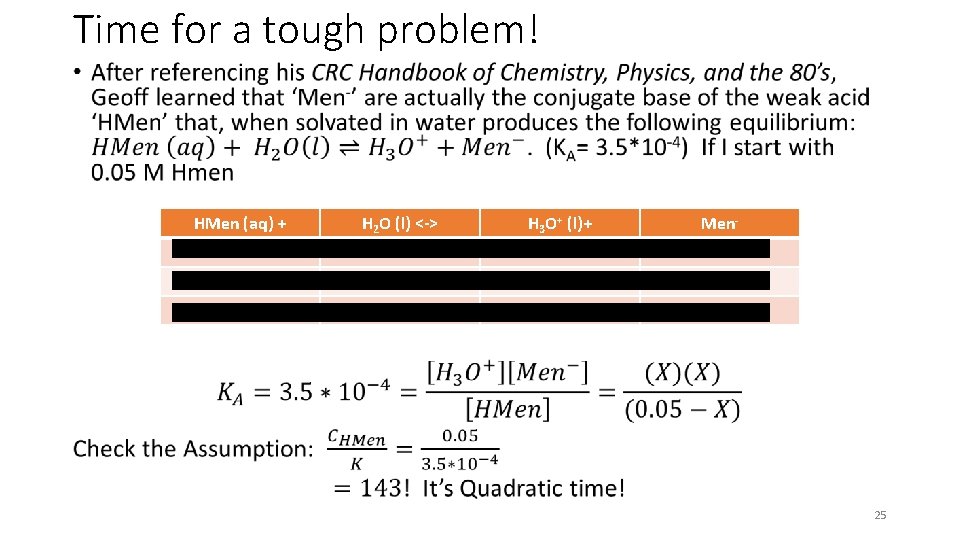
Time for a tough problem! • HMen (aq) + H 2 O (l) <-> H 3 O+ (l)+ Men- 0. 05 M - 0 0 -X - +X +X 0. 05 - X X 25
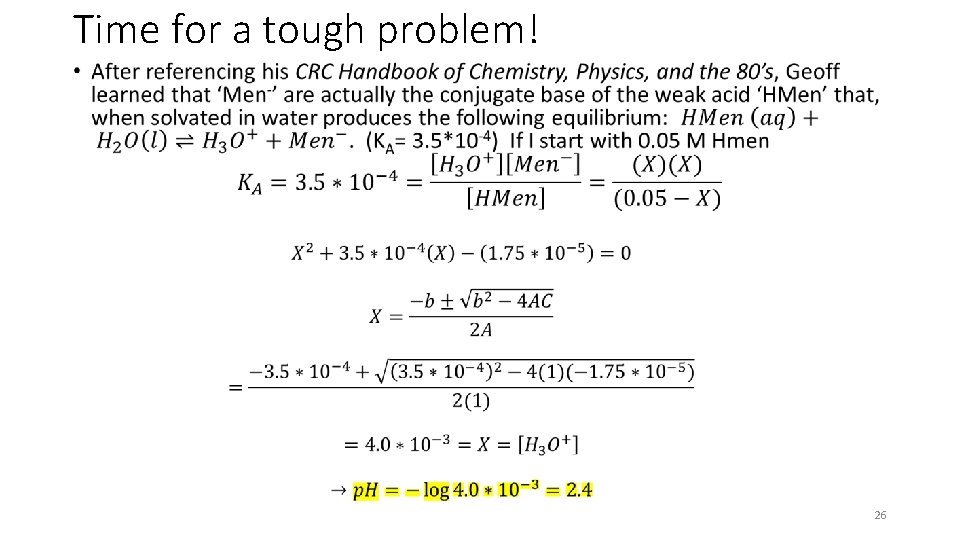
Time for a tough problem! • 26
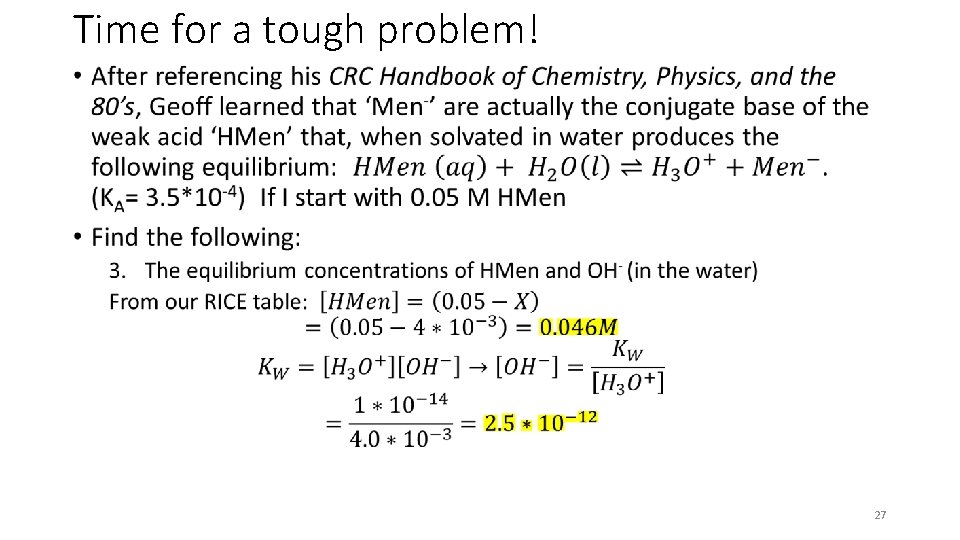
Time for a tough problem! • 27
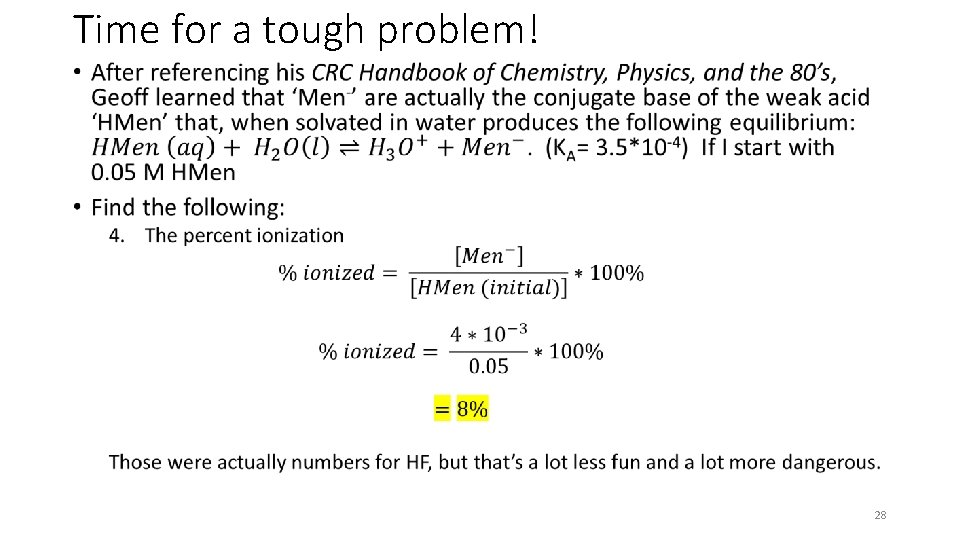
Time for a tough problem! • 28

Good Luck Studying! 29
- Slides: 29