Exam 2 Monday April 16 th Exam Content














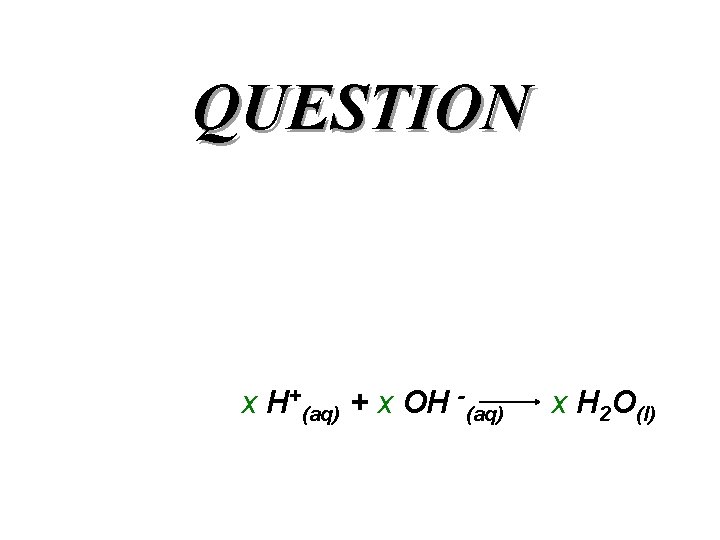
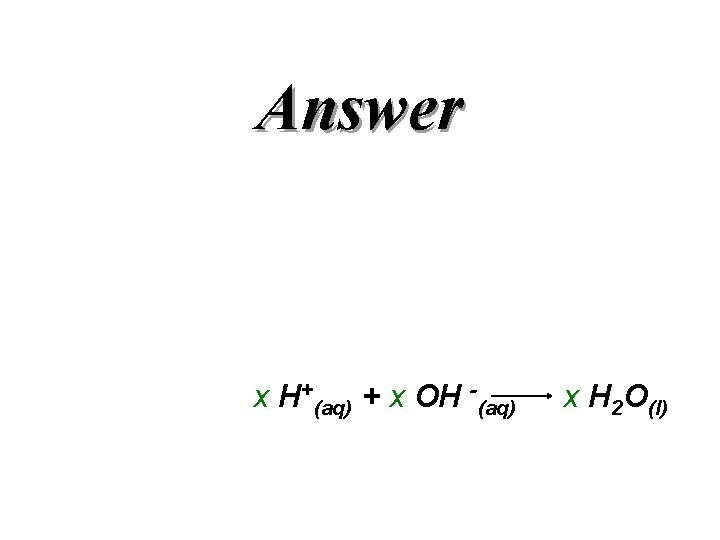





















- Slides: 58

Exam #2: Monday April 16 th Exam Content: Refer to calendar links 5 -Mar through 11 -Apr 20 multiple choice questions (4 pts each), 10 True/False questions (2 pts each) and ~5 -6 problems of various types: matching, fill-in, and applications of mathematical calculations (~4 -6 pts each). The total possible exam points will be ~130 raw points, which will be normalized to 100%. The majority of exam questions will be drawn from Webassign, Guiding Questions & Quizzes, and i-clicker Discussion topics since Exam 1, including those topics not covered on Exam 1, ions: protons, electrons, neutrons. For more specific examples of the types of questions refer to the Calendar’s 16 -Apr link for Practice Questions

Exam #2: Monday April 16 th Scantron will be provided, and a calculator if needed. Cell phones not allowed. Study Guide/ Exam aides: 2 pages, 2 -sided, handwritten notes (Exam 1 + 1), plus Periodic Table handout All materials to be turned in @ end of exam

Aqueous Reactions (Solutions/ Molarity) Net Ionic Equations Dr. Ron Rusay

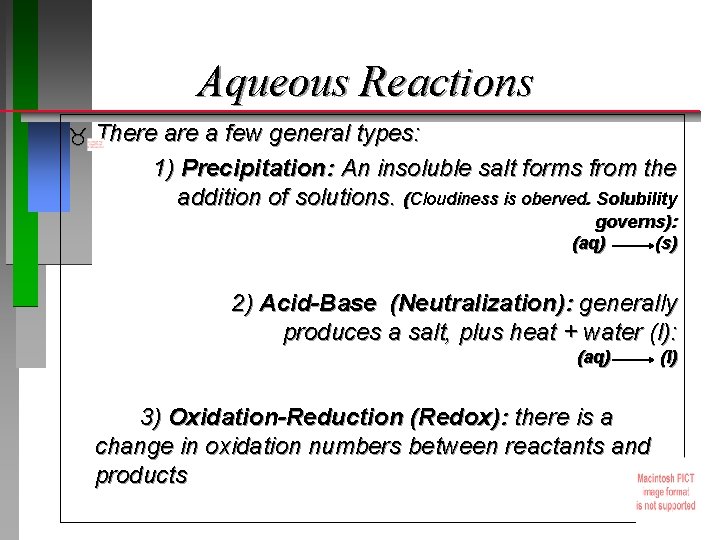
Aqueous Reactions There a few general types: 1) Precipitation: An insoluble salt forms from the addition of solutions. (Cloudiness is oberved. Solubility governs): (aq) ( s) 2) Acid-Base (Neutralization): generally produces a salt, plus heat + water (l): (aq) 3) Oxidation-Reduction (Redox): there is a change in oxidation numbers between reactants and products ( l)

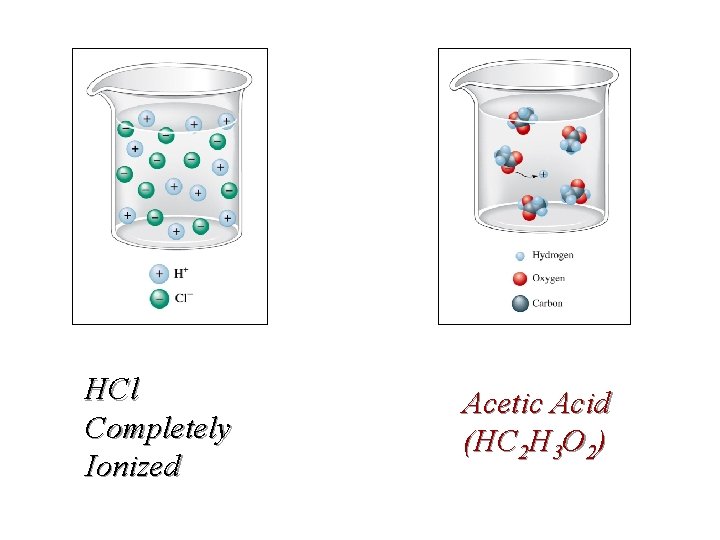
Electrolytes Ions in an aqueous (water) solution Pure Water does not conduct electricity. A water solution must have ions to conduct electricity. Aqueous solutions can be categorized into 3 types: non-electrolytes, strong electrolytes or weak electrolytes based on their ability to conduct electricity in a homogeneous aqueous solution (aq). Aqueous solutions can be tested for conductivity which will determine the degree of ionization of the solute, that is, the substance dissolved in water. It is possible to have full or partial ionization.

Solution Test Apparatus for Electrolytes (Ions) Conductivity depends on the amount of ions in solution strong weak non-

Conductivity http: //chemconnections. org/general/movies/html-swf/electrolytes. htm

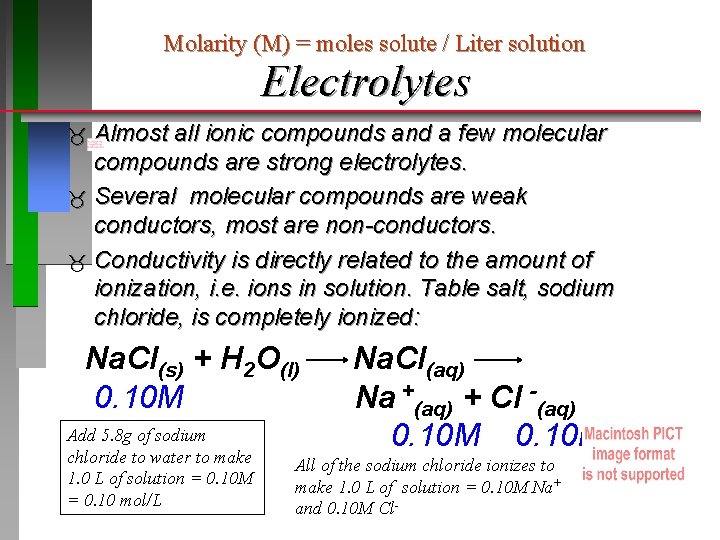
Molarity (M) = moles solute / Liter solution Electrolytes Almost all ionic compounds and a few molecular compounds are strong electrolytes. Several molecular compounds are weak conductors, most are non-conductors. Conductivity is directly related to the amount of ionization, i. e. ions in solution. Table salt, sodium chloride, is completely ionized: Na. Cl(s) + H 2 O(l) 0. 10 M Add 5. 8 g of sodium chloride to water to make 1. 0 L of solution = 0. 10 M = 0. 10 mol/L Na. Cl(aq) Na +(aq) + Cl -(aq) 0. 10 M All of the sodium chloride ionizes to make 1. 0 L of solution = 0. 10 M Na+ and 0. 10 M Cl-

Molarity (M) = moles solute / Liter solution Electrolytes Concentrations: ð Ca. Cl 2 (s) + H 2 O(l) Ca. Cl 2(aq) 0. 10 M Ca 2+(aq) + 2 Cl -(aq) 0. 10 M 0. 20 M ð How many grams of calcium chloride (MM =111 g/mol) should be added to water to make 1. 00 L of a 0. 10 M solution of calcium chloride? How many grams of calcium chloride (MM =111 g/mol) should be added to water to make 1. 00 L of a solution having 0. 10 M chloride ion?

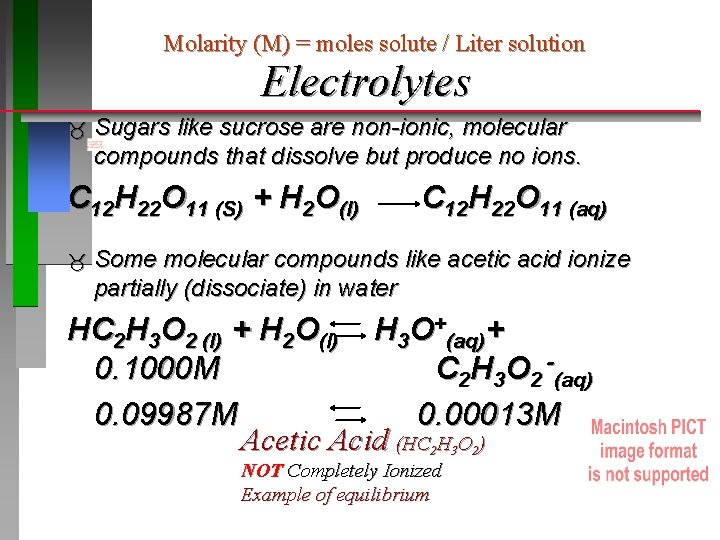
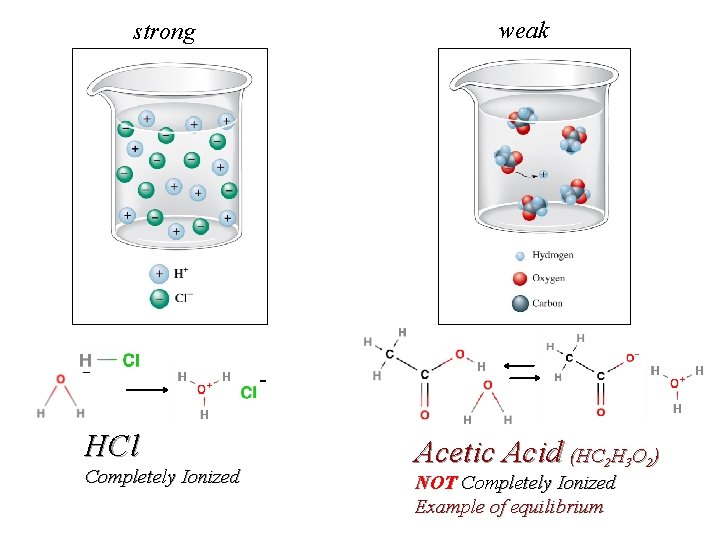
Molarity (M) = moles solute / Liter solution Electrolytes Sugars like sucrose are non-ionic, molecular compounds that dissolve but produce no ions. C 12 H 22 O 11 (S) + H 2 O(l) C 12 H 22 O 11 (aq) Some molecular compounds like acetic acid ionize partially (dissociate) in water HC 2 H 3 O 2 (l) + H 2 O(l) H 3 O+(aq)+ 0. 1000 M C 2 H 3 O 2 -(aq) 0. 09987 M 0. 00013 M Acetic Acid (HC 2 H 3 O 2) NOT Completely Ionized Example of equilibrium

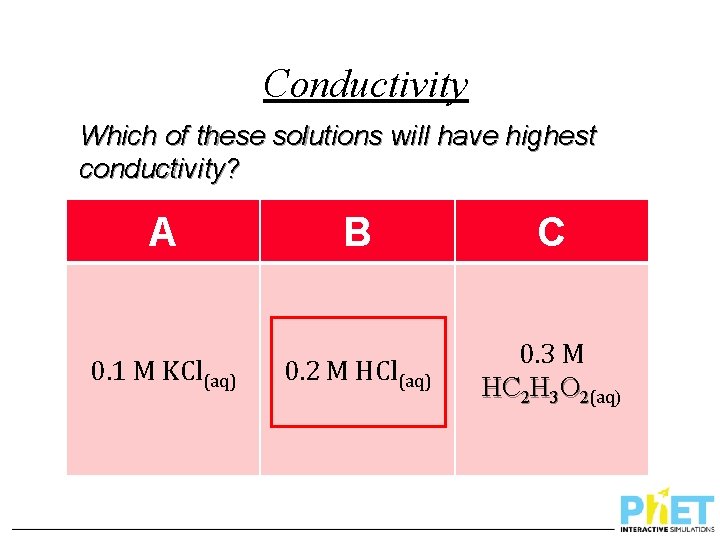
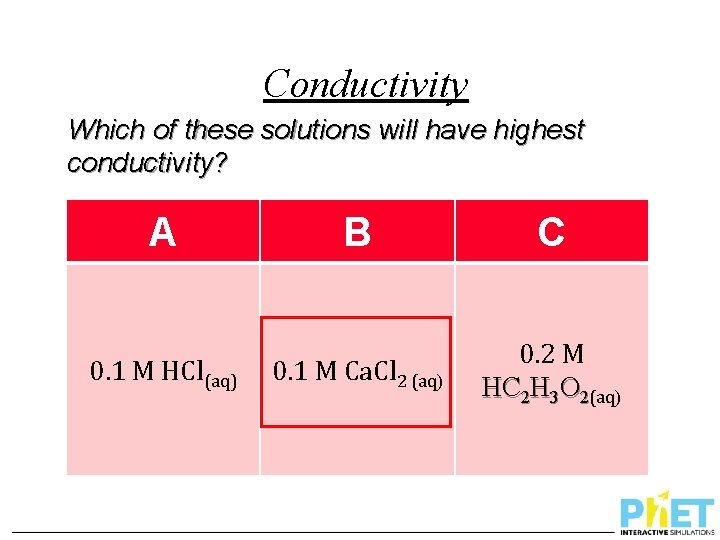
Conductivity Which of these solutions will have highest conductivity? A 0. 1 M KCl(aq) B C 0. 2 M HCl(aq) 0. 3 M HC 2 H 3 O 2(aq)

HCl Completely Ionized Acetic Acid (HC 2 H 3 O 2)

Conductivity Which of these solutions will have highest conductivity? A 0. 1 M HCl(aq) B C 0. 1 M Ca. Cl 2 (aq) 0. 2 M HC 2 H 3 O 2(aq)

Aqueous Acids ð Any compound that provides a proton can be considered an acid. Strong acids are sulfuric acid, nitric acid, perchloric acid, HI, HBr and HCl.

Electrolytes ð How would the conductivity of acetic acid compare to hydrochloric acid?

weak strong - HCl Completely Ionized Acetic Acid (HC 2 H 3 O 2) NOT Completely Ionized Example of equilibrium

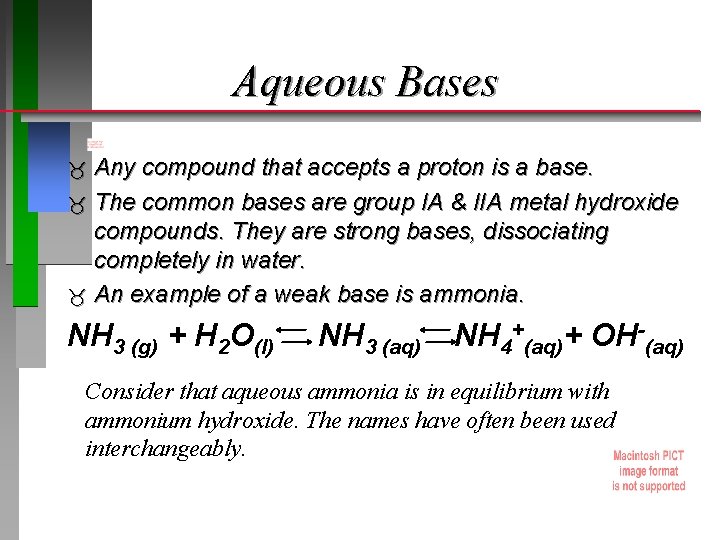
Aqueous Bases Any compound that accepts a proton is a base. The common bases are group IA & IIA metal hydroxide compounds. They are strong bases, dissociating completely in water. An example of a weak base is ammonia. NH 3 (g) + H 2 O(l) NH 3 (aq) NH 4+(aq)+ OH-(aq) Consider that aqueous ammonia is in equilibrium with ammonium hydroxide. The names have often been used interchangeably.

weak strong An Aqueous Solution of Sodium Hydroxide An Aqueous Solution of Ammonia Na. OH (aq) Na+(aq)+ OH-(aq) NH 3 (aq) Completely Ionized NH 4+(aq)+ OH-(aq) NOT Completely Ionized Example of equilibrium

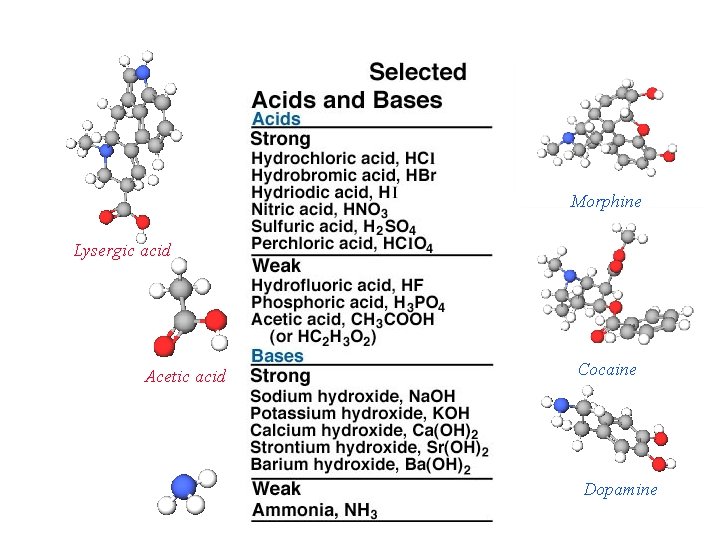
Morphine Lysergic acid Acetic acid Cocaine Dopamine

QUESTION

ANSWER

Aqueous Reactions: Neutralization

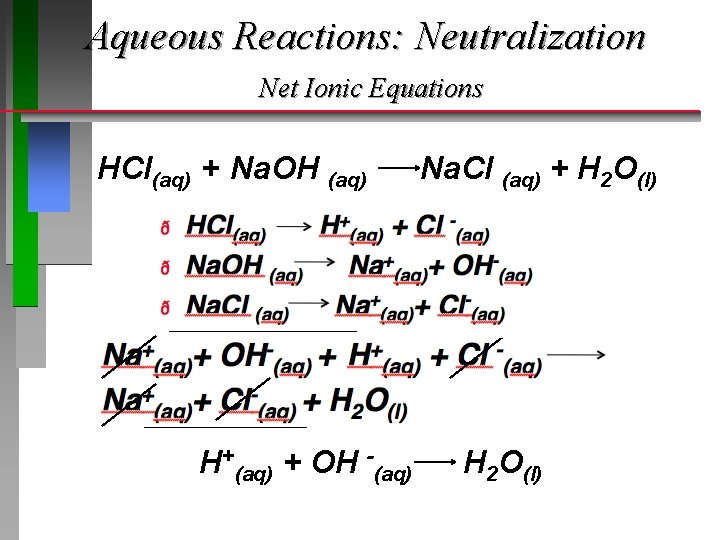
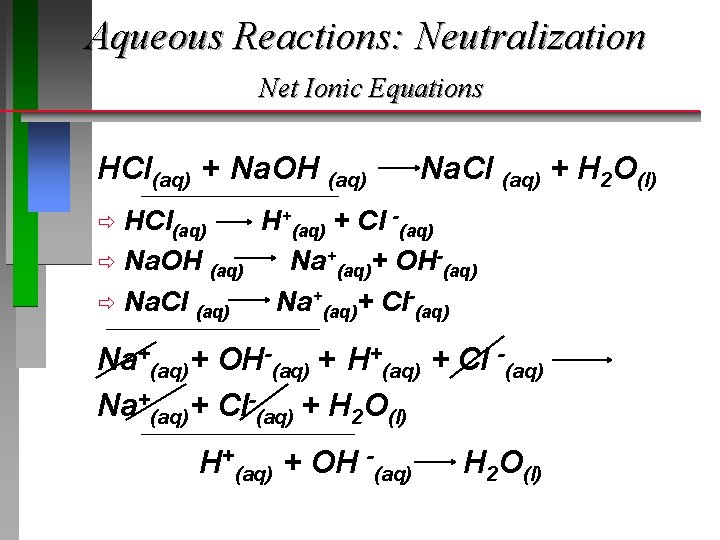
Aqueous Reactions: Neutralization Net Ionic Equations HCl(aq) + Na. OH (aq) H+(aq) + OH -(aq) Na. Cl (aq) + H 2 O(l)

QUESTION

Answer How many moles of sodium hydroxide will it take to completely neutralize 1 mole of sulfuric acid H 2 SO 4? …. to neutralize 1 mole of nitric acid HNO 3?
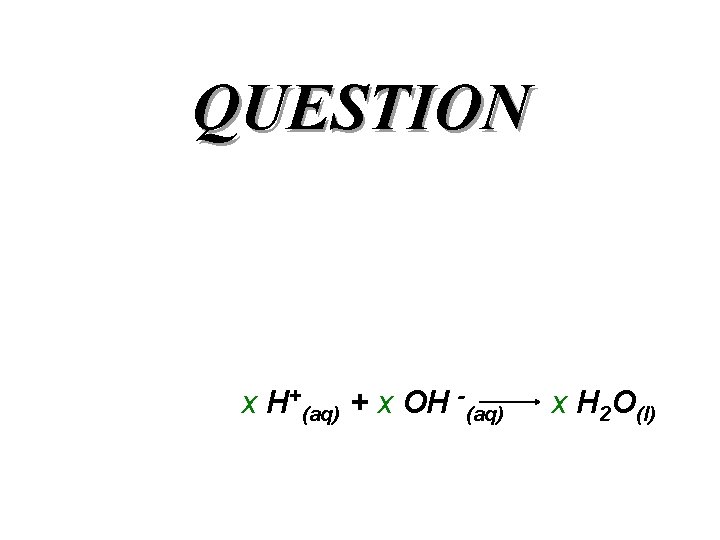
QUESTION x H+(aq) + x OH -(aq) x H 2 O(l)
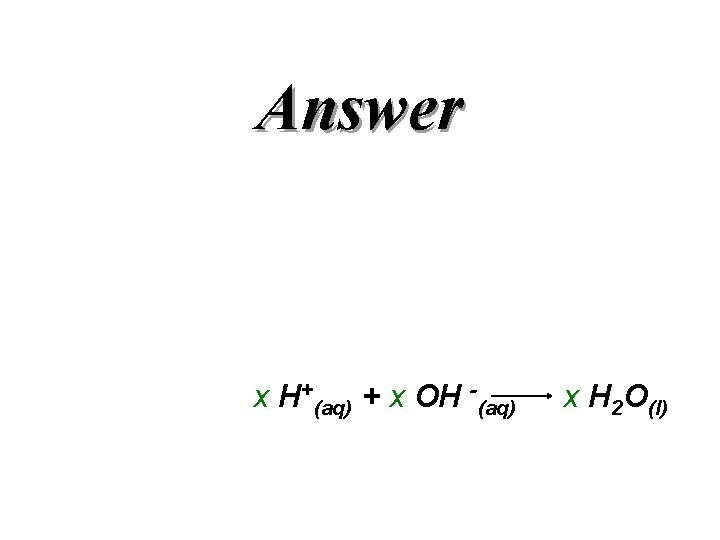
Answer x H+(aq) + x OH -(aq) x H 2 O(l)

Aqueous Reactions: Acid-Base

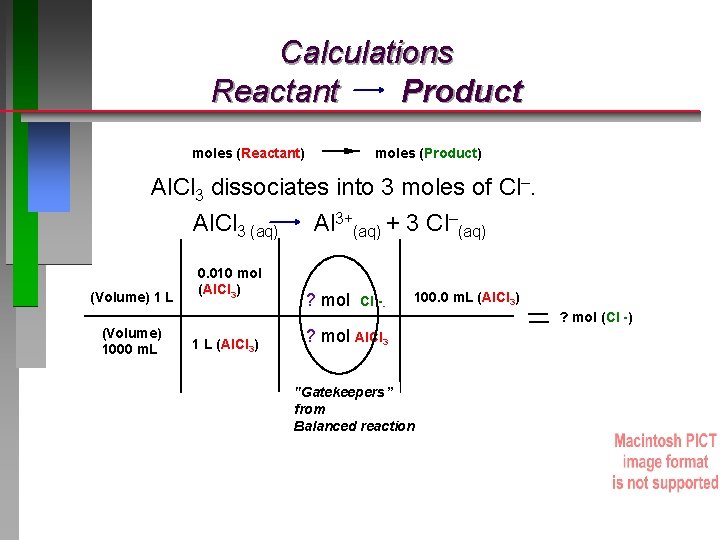
QUESTION If an antacid contains Al(OH)3 it will form Al. Cl 3 upon neutralization of stomach acid. How many moles of Cl– ions are in 100. 0 m. L of 0. 010 M Al. Cl 3? A. 0. 0010 mol B. 0. 010 mol C. 0. 0030 mol D. 0. 030 mol Molarity (M) = mol Al. Cl 3 / Liter solution mol Al. Cl 3 = Molarity Al. Cl 3 x Volume solution (L)

QUESTION If an antacid contains Al(OH)3 it will form Al. Cl 3 upon neutralization of stomach acid. How many moles of Cl– ions are in 100. 0 m. L of 0. 010 M Al. Cl 3? A. 0. 0010 mol B. 0. 010 mol C. 0. 0030 mol D. 0. 030 mol Molarity (M) = moles Al. Cl 3 / Liter solution mol Al. Cl 3 = Molarity Al. Cl 3 x Volume solution (L) Al. Cl 3 dissociates into 3 moles of Cl–.

Calculations Reactant Product moles (Reactant) moles (Product) Al. Cl 3 dissociates into 3 moles of Cl–. Al. Cl 3 (aq) Al 3+(aq) + 3 Cl–(aq) (Volume) 1 L (Volume) 1000 m. L 0. 010 mol (Al. Cl 3) 1 L (Al. Cl 3) ? mol Cl -- 100. 0 m. L (Al. Cl 3) ? mol Al. Cl 3 "Gatekeepers” from Balanced reaction ? mol (Cl -)

ANSWER If an antacid contains Al(OH)3 it will form Al. Cl 3 upon neutralization of stomach acid. How many moles of Cl– ions are in 100. 0 m. L of 0. 010 M Al. Cl 3? A. 0. 0010 mol B. 0. 010 mol C. 0. 0030 mol D. 0. 030 mol Al. Cl 3 (aq) (Volume) 1 L (Volume) 1000 m. L Al 3+(aq) + 3 Cl–(aq) 0. 010 mol (Al. Cl 3) 1 L (Al. Cl 3) 3 mol Cl -- 100. 0 m. L (Al. Cl 3) 1 mol Al. Cl 3 0. 0030 mol (Cl -) "Gatekeepers” from Balanced reaction Molarity (M) = moles solute / Liter solution

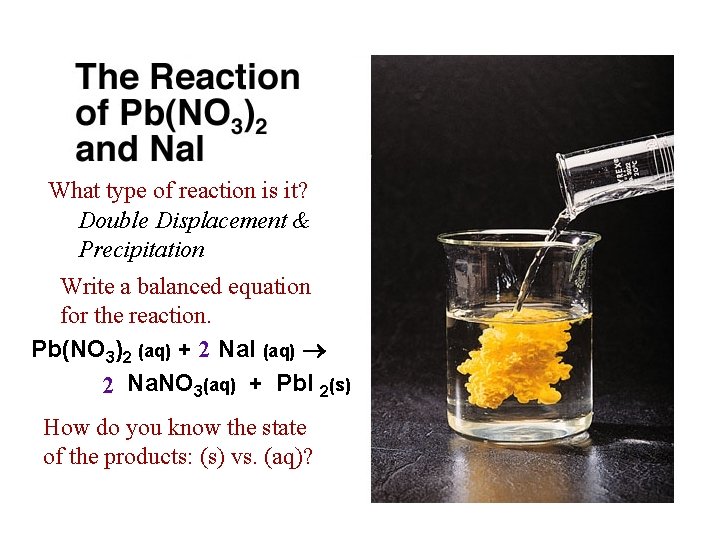
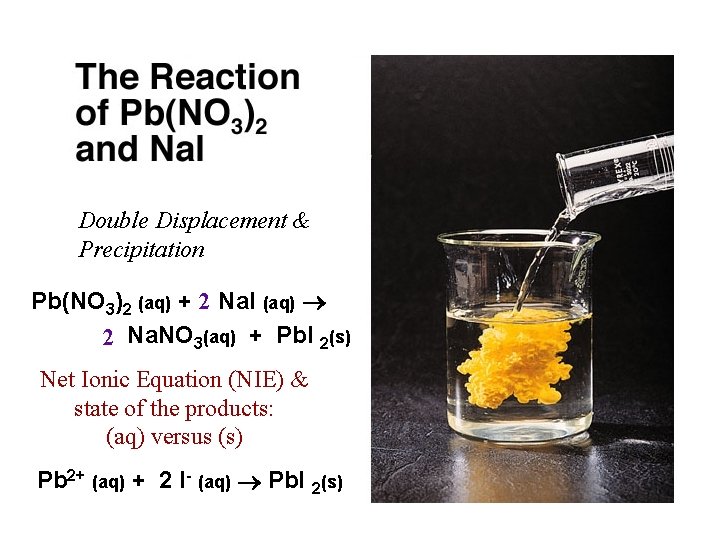
What type of reaction is it? Double Displacement & Precipitation Write a balanced equation for the reaction. Pb(NO 3)2 (aq) + 2 Na. I (aq) 2 Na. NO 3(aq) + Pb. I 2(s) How do you know the state of the products: (s) vs. (aq)?

Exam #2: Monday April 16 th Includes (GQ) Solutions & Concentrations Guiding Questions 20 multiple choice questions (4 pts each), 10 True/False questions (2 pts each) and ~5 -6 problems of various types: matching, fill-in, and applications of mathematical calculations (~4 -6 pts each). The total possible exam points will be ~130 raw points, which will be normalized to 100%. For specific examples of the types of questions refer to the Calendar’s 16 -Apr link for Practice Questions Study Guide/ Exam aides: 2 pages, 2 -sided, handwritten notes (Exam 1 + 1), plus Periodic Table handout

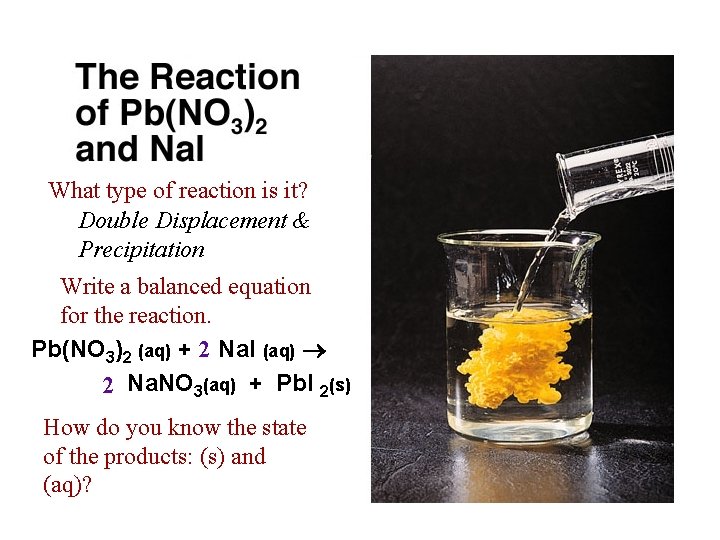
What type of reaction is it? Double Displacement & Precipitation Write a balanced equation for the reaction. Pb(NO 3)2 (aq) + 2 Na. I (aq) 2 Na. NO 3(aq) + Pb. I 2(s) How do you know the state of the products: (s) and (aq)?

Precipitation Reactions: Solubility Tables (aq) soluble versus (s) insoluble

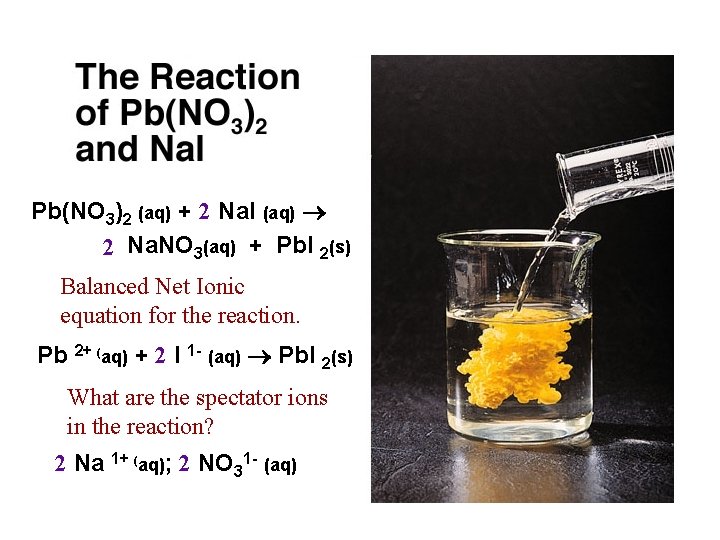
Double Displacement & Precipitation Pb(NO 3)2 (aq) + 2 Na. I (aq) 2 Na. NO 3(aq) + Pb. I 2(s) Net Ionic Equation (NIE) & state of the products: (aq) versus (s) Pb 2+ (aq) + 2 I- (aq) Pb. I 2(s)

Pb(NO 3)2 (aq) + 2 Na. I (aq) 2 Na. NO 3(aq) + Pb. I 2(s) Balanced Net Ionic equation for the reaction. Pb 2+ (aq) + 2 I 1 - (aq) Pb. I 2(s) What are the spectator ions in the reaction? 2 Na 1+ (aq); 2 NO 31 - (aq)

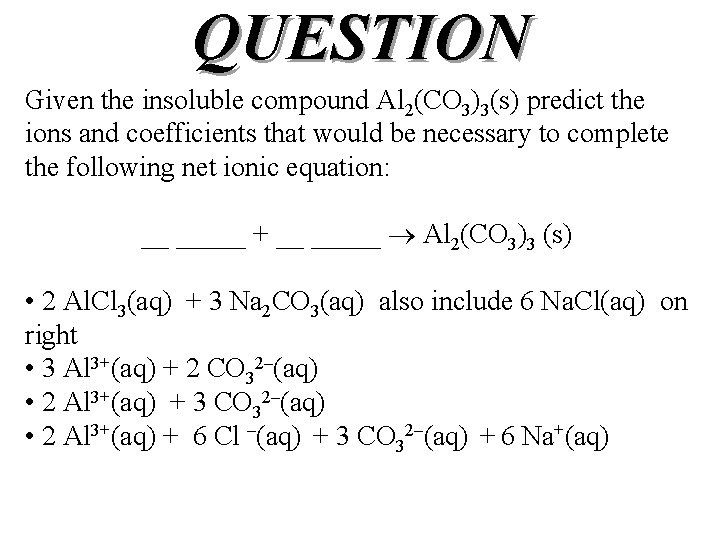
QUESTION Given the insoluble compound Al 2(CO 3)3(s) predict the ions and coefficients that would be necessary to complete the following net ionic equation: __ _____ + __ _____ Al 2(CO 3)3 (s) • 2 Al. Cl 3(aq) + 3 Na 2 CO 3(aq) also include 6 Na. Cl(aq) on right • 3 Al 3+(aq) + 2 CO 32–(aq) • 2 Al 3+(aq) + 3 CO 32–(aq) • 2 Al 3+(aq) + 6 Cl –(aq) + 3 CO 32–(aq) + 6 Na+(aq)

ANSWER Given the insoluble compound Al 2(CO 3)3 predict the ions and coefficients that would be necessary to complete the following net ionic equation: __ _____ + __ _____ Al 2(CO 3)3 (s) • 2 Al. Cl 3(aq) + 3 Na 2 CO 3(aq) also include 6 Na. Cl(aq) on right • 3 Al 3+(aq) + 2 CO 32–(aq) • 2 Al 3+(aq) + 3 CO 32–(aq) • 2 Al 3+(aq) + 6 Cl –(aq) + 3 CO 32–(aq) + 6 Na+(aq)

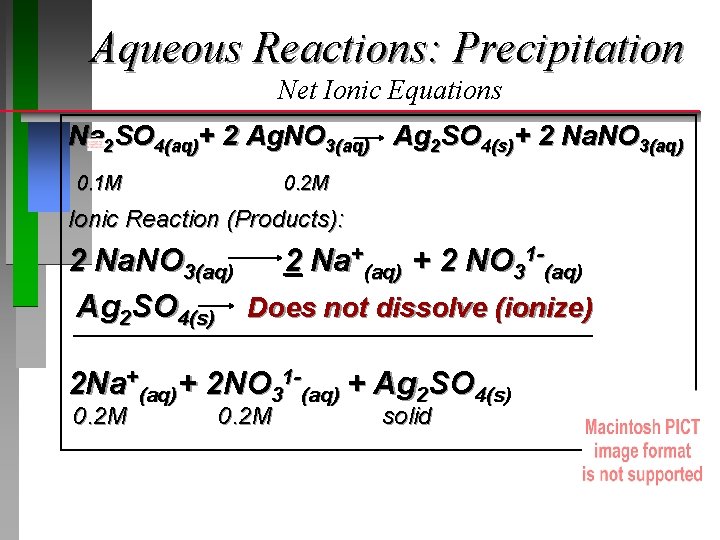
Aqueous Reactions: Precipitation Net Ionic Equations 50 m. L of a 0. 1 M solution of sodium sulfate is mixed with 50 m. L of a 0. 2 M solution of silver nitrate. What is the result? Molecular Equation: 1 2

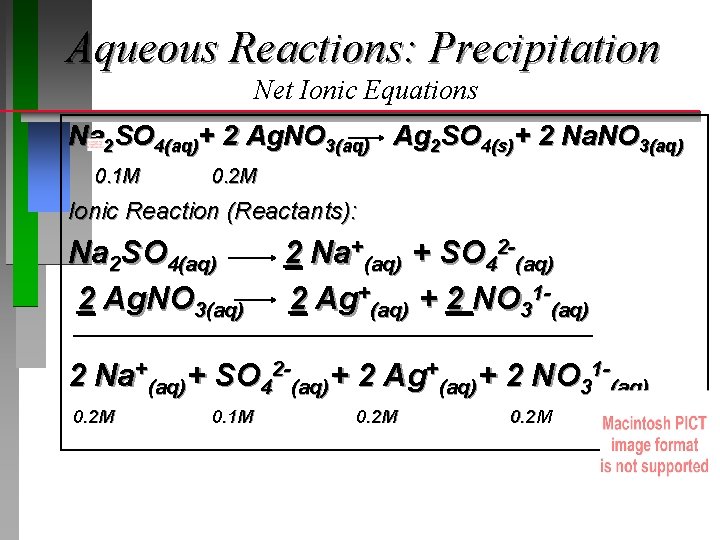
Aqueous Reactions: Precipitation Net Ionic Equations Na 2 SO 4(aq)+ 2 Ag. NO 3(aq) Ag 2 SO 4(s)+ 2 Na. NO 3(aq) 0. 1 M 0. 2 M Ionic Reaction (Reactants): Na 2 SO 4(aq) 2 Ag. NO 3(aq) 2 Na+(aq) + SO 42 -(aq) 2 Ag+(aq) + 2 NO 31 -(aq) 2 Na+(aq)+ SO 42 -(aq)+ 2 Ag+(aq)+ 2 NO 31 -(aq) 0. 2 M 0. 1 M 0. 2 M

Aqueous Reactions: Precipitation Net Ionic Equations Na 2 SO 4(aq)+ 2 Ag. NO 3(aq) Ag 2 SO 4(s)+ 2 Na. NO 3(aq) 0. 1 M 0. 2 M Ionic Reaction (Products): 2 Na. NO 3(aq) 2 Na+(aq) + 2 NO 31 -(aq) Ag 2 SO 4(s) Does not dissolve (ionize) 2 Na+(aq)+ 2 NO 31 -(aq) + Ag 2 SO 4(s) 0. 2 M solid

Aqueous Reactions: Precipitation Net Ionic Equations Na 2 SO 4(aq)+ 2 Ag. NO 3(aq) Ag 2 SO 4(s)+ 2 Na. NO 3(aq) Overall Ionic Reaction: 2 Na+(aq)+ SO 42 -(aq) +2 Ag+(aq)+ 2 NO 31 -(aq) 2 Na+(aq) + Ag 2 SO 4(s) + 2 NO 31 -(aq) Net Ionic Equation: (Subtract Spectator Ions) 2 Ag+(aq)+ SO 42 -(aq) MSO 42 -(aq) x Vsolution= mol = 0. 10 mol/L x 0. 050 L = 0. 0050 mol How many moles 4(s) form? Ag 2 SO = MNa 2 SO 4 x VNa 2 SO 4 / 1: 1 stoichiometry = 0. 0050 mol Ag 2 SO 4(s)

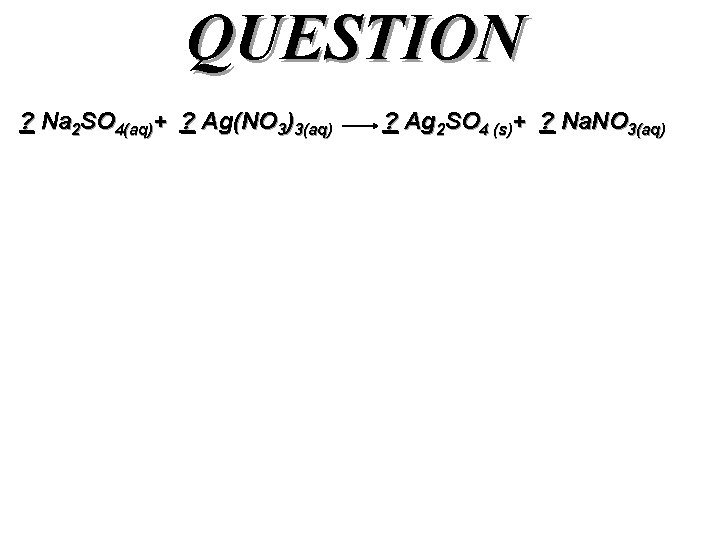
QUESTION ? Na 2 SO 4(aq)+ ? Ag(NO 3)3(aq) ? Ag 2 SO 4 (s)+ ? Na. NO 3(aq)

Answer 1 Na 2 SO 4(aq)+ 2 Ag(NO 3)3(aq) SO 42 - (aq)+ 2 Ag+ (aq) 1 Ag 2 SO 4 (s)+ 2 Na. NO 3(aq) Ag 2 SO 4 (s)

QUESTION & Answer

QUESTION & ANSWER If you began a reaction with the following ions in solution (all would be written with an (aq) subscript how would you represent the proper final net ionic equation? (Consult a solubility Table. ) 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– • • 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– No Reaction 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– Fe 3(PO 4)2 (s)+ 6 Na. NO 3 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– Fe 3(PO 4)2 (s)+ 6 Na+ + 6 NO 3– 2 PO 43– + 3 Fe 2+ Fe 3(PO 4)2 (s)


ANSWER –

QUESTION

ANSWER E) All of these are soluble in water. According to the solubility rules for ionic compounds, compounds containing Group IA ions or nitrate ions will always be soluble. Compounds containing halides are generally soluble, aside from silver, lead and mercury(I) halides.

QUESTION If you began a reaction with the following ions in solution (all would be written with an (aq) subscript how would you represent the proper final net ionic equation? (Consult solubility Table. ) 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– • 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– No Reaction • 6 Na+ + 2 PO 43– + 3 Fe 2+ + 6 NO 3– Fe 3(PO 4)2 (s)+ 6 Na. NO 3 • 3 Na+ + PO 43– + Fe 2+ + 2 NO 3– Fe 3(PO 4)2 (s)+ 6 Na+ + 6 NO 3– • 2 PO 43– + 3 Fe 2+ Fe 3(PO 4)2 (s)

QUESTION An aqueous solution of H 2 SO 4 is added to aqueous Ba(OH)2. The reaction is monitored using a conductivity tester. Predict the correct statement(s). I) Both H 2 SO 4 and Ba(OH)2 are strong electrolytes. II) This is a neutralization reaction. III) This is a precipitation reaction. IV) The light bulb will glow at the neutralization point. A) II B) I and II C) I, II and III D) I, III and IV

ANSWER An aqueous solution of H 2 SO 4 is added to aqueous Ba(OH)2. The reaction is monitored using a conductivity tester. Predict the correct statement(s). I) Both H 2 SO 4 and Ba(OH)2 are strong electrolytes. II) This is a neutralization reaction. III) This is a precipitation reaction. IV) The light bulb will glow at the neutralization point. A) II B) I and II C) I, II and III D) I, III and IV

Aqueous Reactions: Neutralization Net Ionic Equations HCl(aq) + Na. OH (aq) __________________________ Na. Cl (aq) + H 2 O(l) HCl(aq) H+(aq) + Cl -(aq) ð Na. OH (aq) Na+(aq)+ OH-(aq) ð Na. Cl (aq) Na+(aq)+ Cl-(aq) ð ________________________ Na+(aq)+ OH-(aq) + H+(aq) + Cl -(aq) Na+(aq)+ Cl-(aq) + H 2 O(l) ____________________________ H+(aq) + OH -(aq) H 2 O(l)

Aqueous Reactions: Precipitation Net Ionic Equations 50 m. L of a 0. 1 M solution of sodium sulfate is mixed with 50 m. L of a 0. 2 M solution of silver nitrate. What is the result? Molecular Equation: ? Na 2 SO 4(aq)+ ? Ag. NO 3(aq) 1 2 1 ? Ag 2 SO 4(s) + ? Na. NO 3(aq) 2

2 PO 43– + Al 3+ Al(OH)3 (s) QUESTION & ANSWER –