EVPP 110 Lab Molecules Process of Life Activities
EVPP 110 Lab Molecules & Process of Life (Activities 3 - 5) Week of February 12 th, 2018
Molecules & Process of Life – Activity 3 – DNA Extraction and Damage by a Pollutant
�What �Why is DNA? is it important? �Where is it located in the cell? �Do all organisms have it?
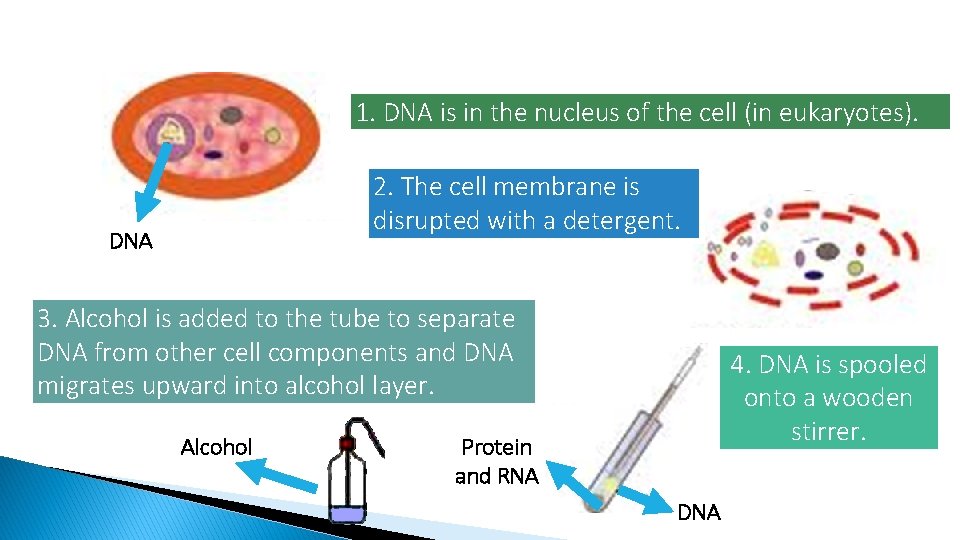
1. DNA is in the nucleus of the cell (in eukaryotes). 2. The cell membrane is disrupted with a detergent. DNA 3. Alcohol is added to the tube to separate DNA from other cell components and DNA migrates upward into alcohol layer. Alcohol 4. DNA is spooled onto a wooden stirrer. Protein and RNA DNA

� Soak strawberry in simulated pollutant. � Mash strawberry in plastic bag and mix with DNA extraction buffer. Wait 5 minutes � Filter mashed mixture into tube. � Add enzymes (contact lens solution. ) Wait 2 minutes � Add cold alcohol. � It will form a layer at the top and DNA will rise. � DNA can be observed and spooled onto a wooden stirrer (do not stir – just twist). Cheesecloth Filter
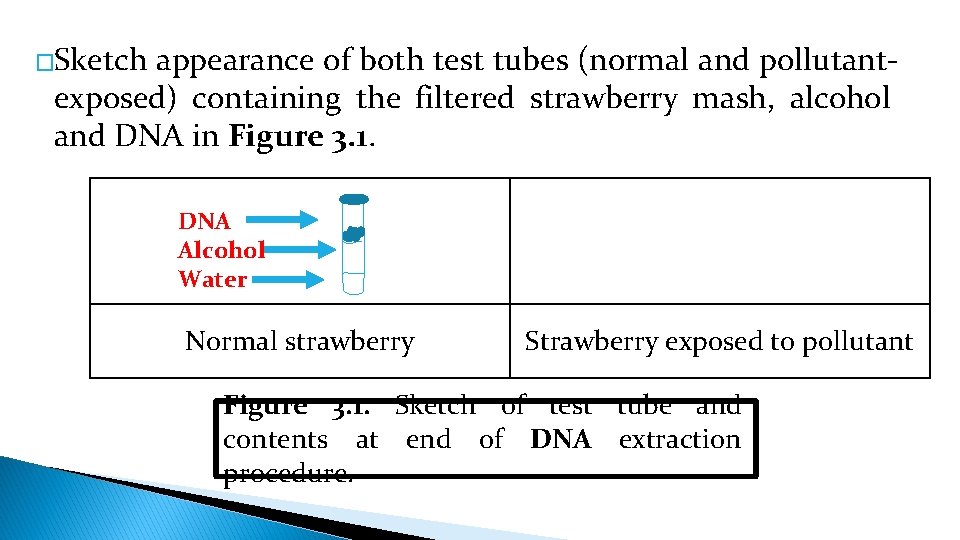
�Sketch appearance of both test tubes (normal and pollutantexposed) containing the filtered strawberry mash, alcohol and DNA in Figure 3. 1. DNA Alcohol Water Normal strawberry Strawberry exposed to pollutant Figure 3. 1. Sketch of test tube and contents at end of DNA extraction procedure.
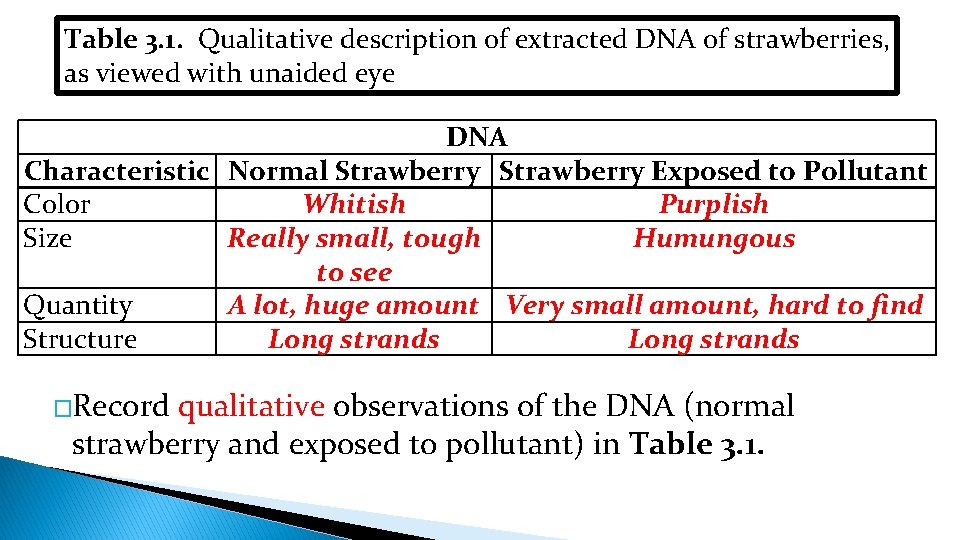
Table 3. 1. Qualitative description of extracted DNA of strawberries, as viewed with unaided eye DNA Characteristic Normal Strawberry Exposed to Pollutant Color Whitish Purplish Size Really small, tough Humungous to see Quantity A lot, huge amount Very small amount, hard to find Structure Long strands �Record qualitative observations of the DNA (normal strawberry and exposed to pollutant) in Table 3. 1.
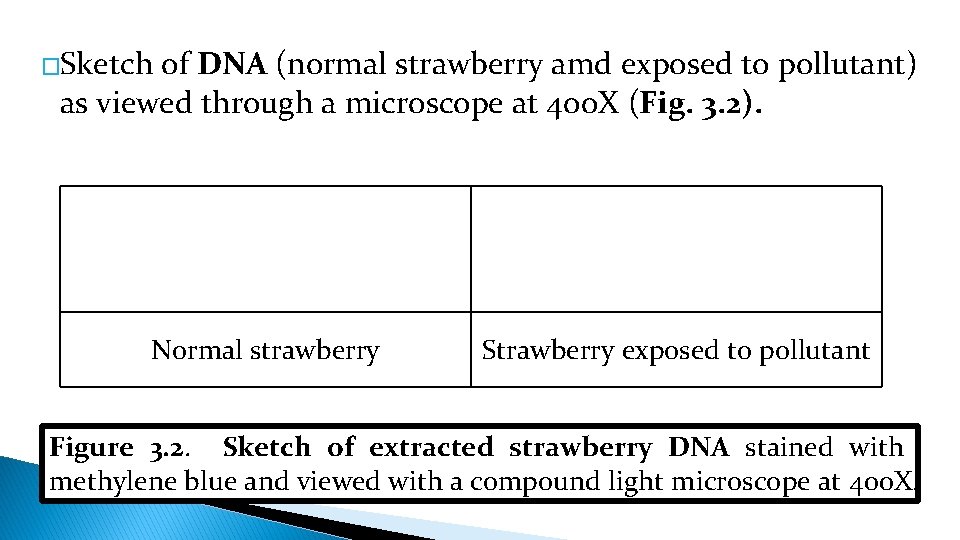
�Sketch of DNA (normal strawberry amd exposed to pollutant) as viewed through a microscope at 400 X (Fig. 3. 2). Normal strawberry Strawberry exposed to pollutant Figure 3. 2. Sketch of extracted strawberry DNA stained with methylene blue and viewed with a compound light microscope at 400 X.
Molecules & Process of Life – Activity 4 – Diffusion and Osmosis – Part A
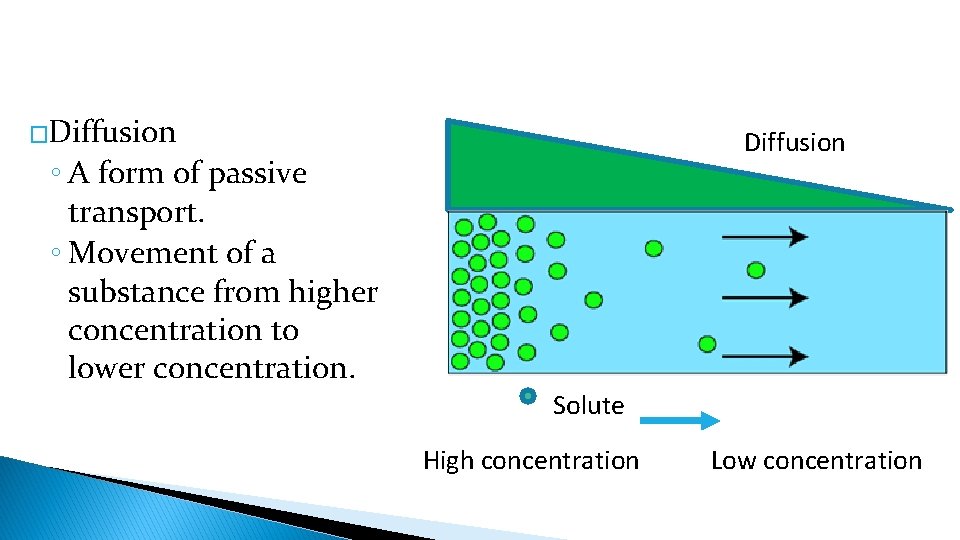
�Diffusion ◦ A form of passive transport. ◦ Movement of a substance from higher concentration to lower concentration. Solute High concentration Low concentration
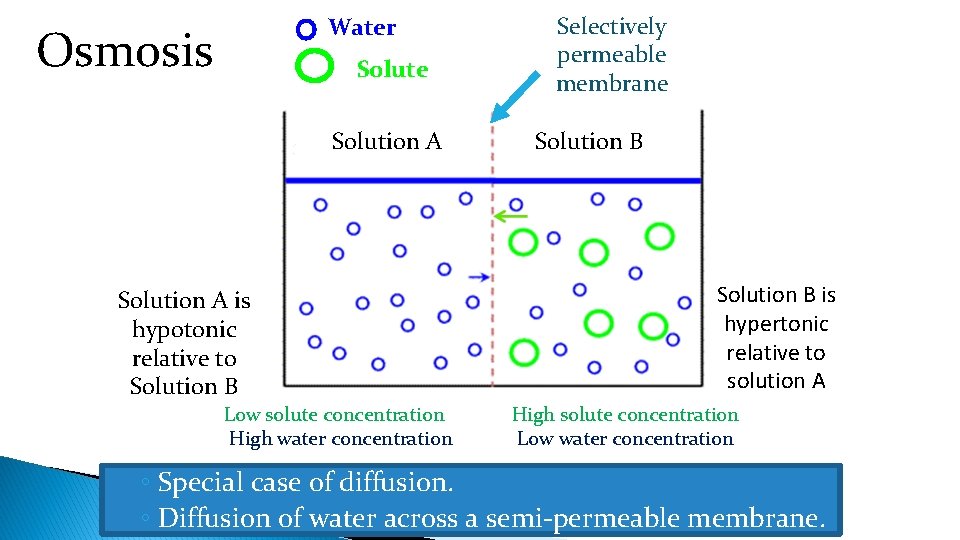
Water Osmosis Solute Solution A is hypotonic relative to Solution B Low solute concentration High water concentration Selectively permeable membrane Solution B is hypertonic relative to solution A High solute concentration Low water concentration ◦ Special case of diffusion. ◦ Diffusion of water across a semi-permeable membrane.

�Dialysis tubing ◦ Comes flat in a roll. ◦ To “open” your piece into a tube, hold it under running water and rub it between thumb and index finger.

�Starch indicator solution ◦ Iodine-based. ◦ In absence of starch = “-” �Amber/yellowish color. ◦ In presence of starch = “+” �Inky/blue-black color.
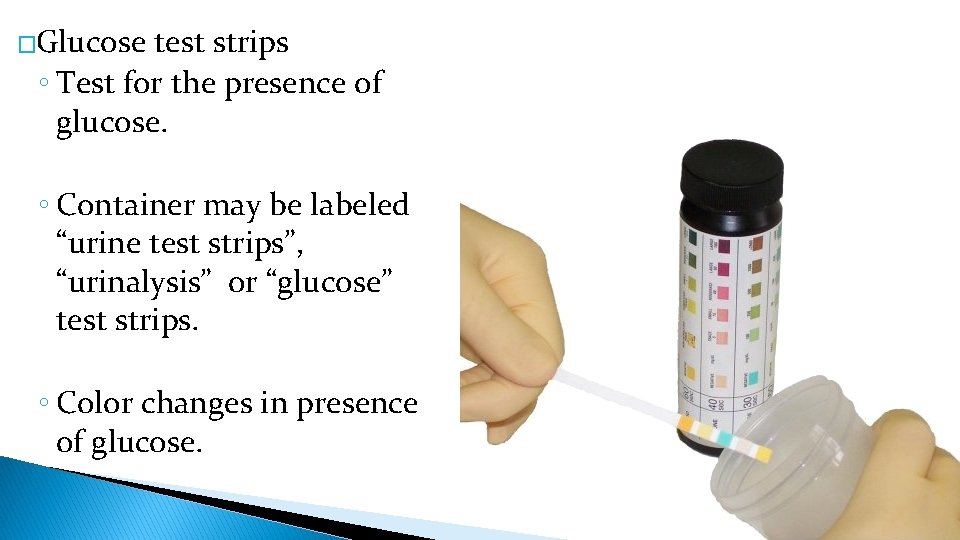
�Glucose test strips ◦ Test for the presence of glucose. ◦ Container may be labeled “urine test strips”, “urinalysis” or “glucose” test strips. ◦ Color changes in presence of glucose.
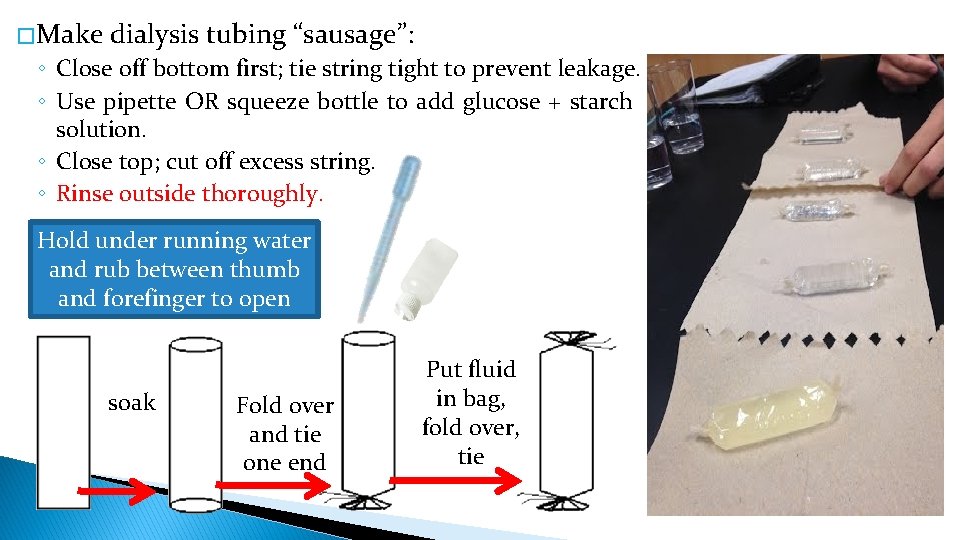
� Make dialysis tubing “sausage”: ◦ Close off bottom first; tie string tight to prevent leakage. ◦ Use pipette OR squeeze bottle to add glucose + starch solution. ◦ Close top; cut off excess string. ◦ Rinse outside thoroughly. Hold under running water and rub between thumb and forefinger to open soak Fold over and tie one end Put fluid in bag, fold over, tie
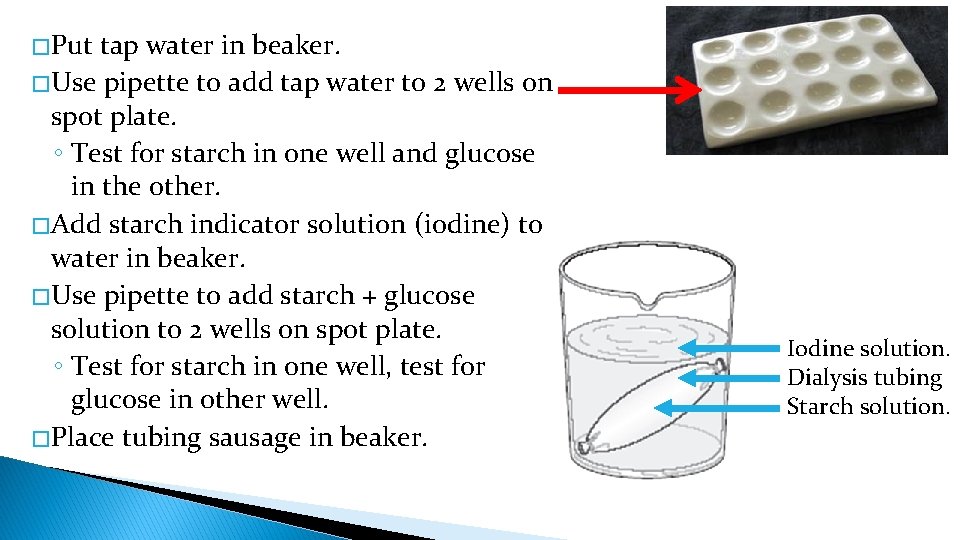
� Put tap water in beaker. � Use pipette to add tap water to 2 wells on spot plate. ◦ Test for starch in one well and glucose in the other. � Add starch indicator solution (iodine) to water in beaker. � Use pipette to add starch + glucose solution to 2 wells on spot plate. ◦ Test for starch in one well, test for glucose in other well. � Place tubing sausage in beaker. Iodine solution. Dialysis tubing Starch solution.
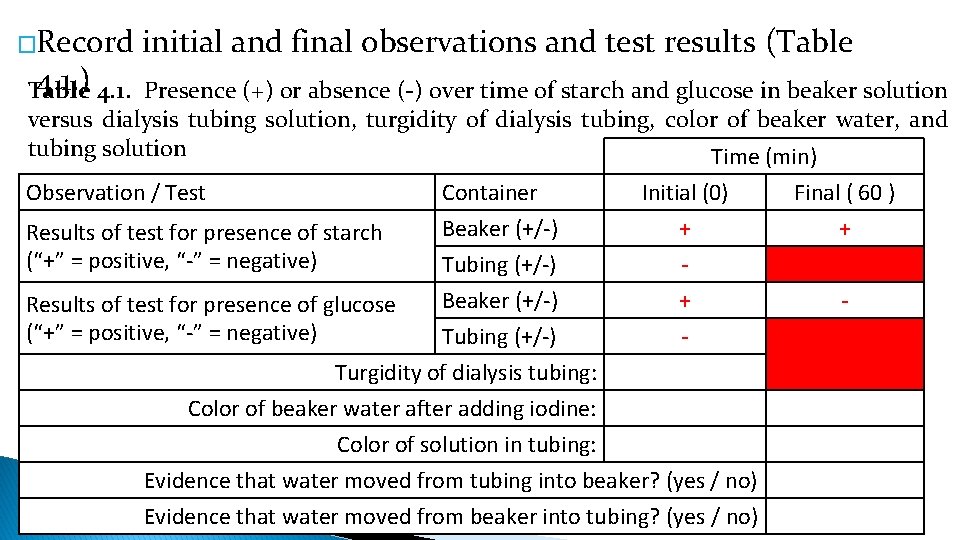
�Record initial and final observations and test results (Table 4. 1. ) 4. 1. Presence (+) or absence (-) over time of starch and glucose in beaker solution Table versus dialysis tubing solution, turgidity of dialysis tubing, color of beaker water, and tubing solution Time (min) Observation / Test Results of test for presence of starch (“+” = positive, “-” = negative) Container Beaker (+/-) Tubing (+/-) Initial (0) + - Beaker (+/-) + Tubing (+/-) Turgidity of dialysis tubing: Color of beaker water after adding iodine: Color of solution in tubing: Evidence that water moved from tubing into beaker? (yes / no) Evidence that water moved from beaker into tubing? (yes / no) Results of test for presence of glucose (“+” = positive, “-” = negative) Final ( 60 ) + -
Molecules & Process of Life – Activity 4 – Diffusion and Osmosis – Part B
�Osmosis: ◦ Special case of diffusion. ◦ Diffusion of water across a semi-permeable membrane �Driven by differences in concentrations of water and solutes on either side of membranes. �Hypertonic �Isotonic �Hypotonic
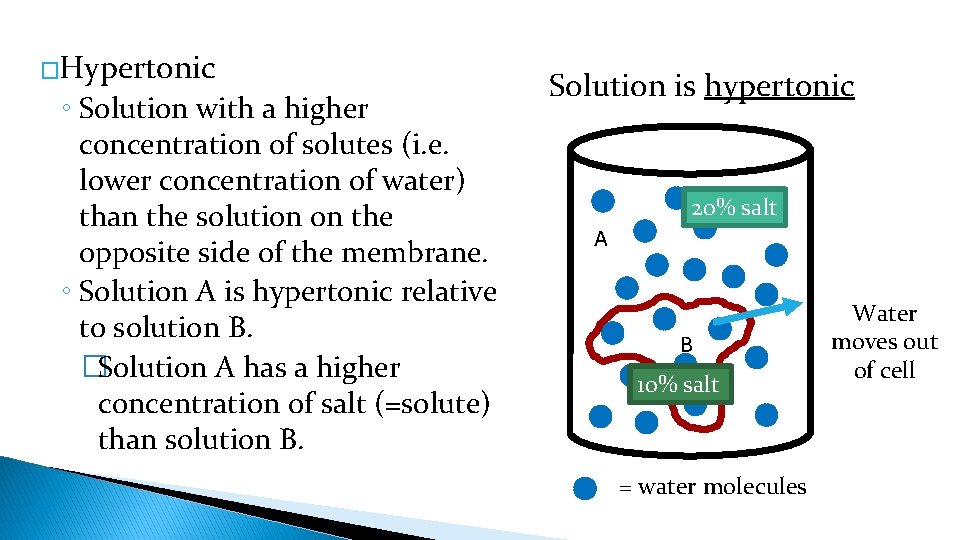
�Hypertonic ◦ Solution with a higher concentration of solutes (i. e. lower concentration of water) than the solution on the opposite side of the membrane. ◦ Solution A is hypertonic relative to solution B. �Solution A has a higher concentration of salt (=solute) than solution B. Solution is hypertonic 20% salt A B 10% salt = water molecules Water moves out of cell
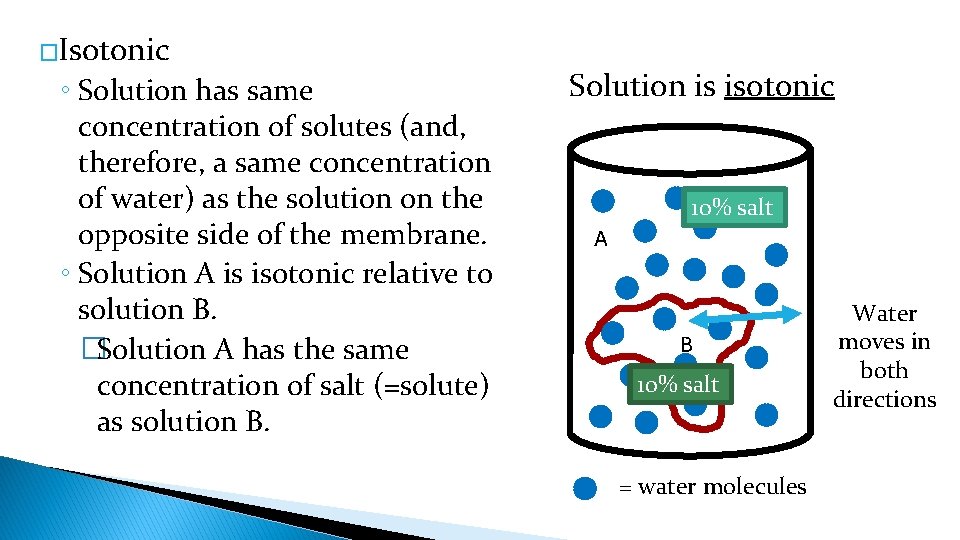
�Isotonic ◦ Solution has same concentration of solutes (and, therefore, a same concentration of water) as the solution on the opposite side of the membrane. ◦ Solution A is isotonic relative to solution B. �Solution A has the same concentration of salt (=solute) as solution B. Solution is isotonic 10% salt A B 10% salt = water molecules Water moves in both directions
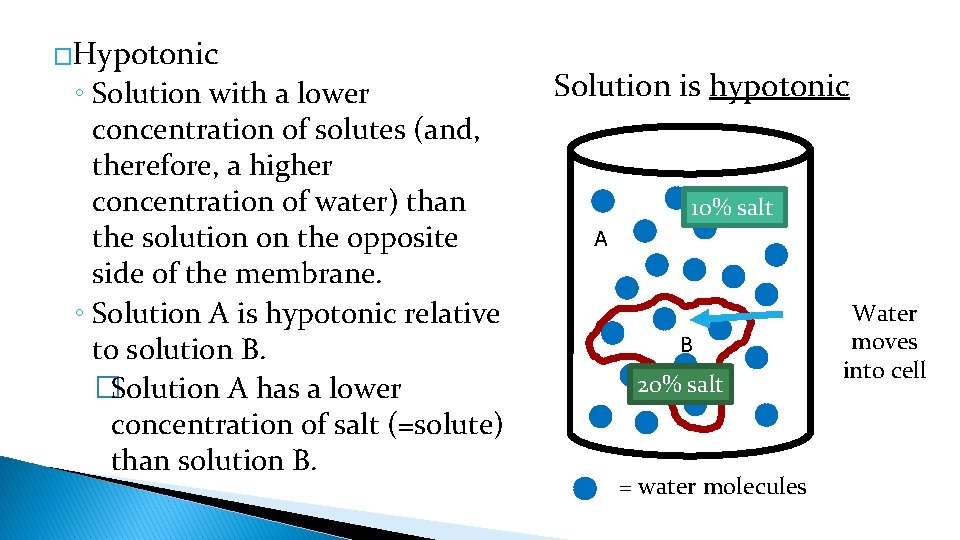
�Hypotonic ◦ Solution with a lower concentration of solutes (and, therefore, a higher concentration of water) than the solution on the opposite side of the membrane. ◦ Solution A is hypotonic relative to solution B. �Solution A has a lower concentration of salt (=solute) than solution B. Solution is hypotonic 10% salt A B 20% salt = water molecules Water moves into cell
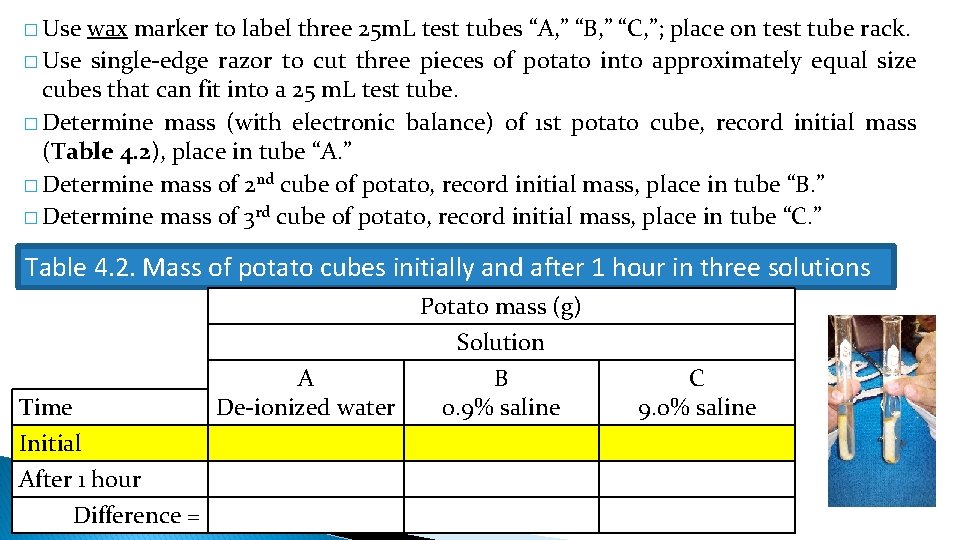
� Use wax marker to label three 25 m. L test tubes “A, ” “B, ” “C, ”; place on test tube rack. � Use single-edge razor to cut three pieces of potato into approximately equal size cubes that can fit into a 25 m. L test tube. � Determine mass (with electronic balance) of 1 st potato cube, record initial mass (Table 4. 2), place in tube “A. ” � Determine mass of 2 nd cube of potato, record initial mass, place in tube “B. ” � Determine mass of 3 rd cube of potato, record initial mass, place in tube “C. ” Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour Difference = A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline
◦ Tube “A”: de-ionized water. ◦ Tube “B”: 0. 9% saline solution. ◦ Tube “C”: 9% saline solution. ◦ Add enough liquid to cover the potato cube! Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour Difference = A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline
�After 1 hour: ◦ Remove potato cube from tube “A, ” pat dry with paper towel, record mass (Table 4. 2). ◦ Repeat for potato cubes in tubes “B” and “C”. Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour Difference = A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline
� Determine and record the difference between the initial mass and the mass “after 1 hour” for the potato cube in each solution (Table 4. 2). Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour Difference = A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline
Molecules and Processes of Life – Activity 5 – Photosynthesis and the Role of Environmental Parameters

The instructions, data sheets, and write-ups are posted in a file in the lab section on Black. Board.
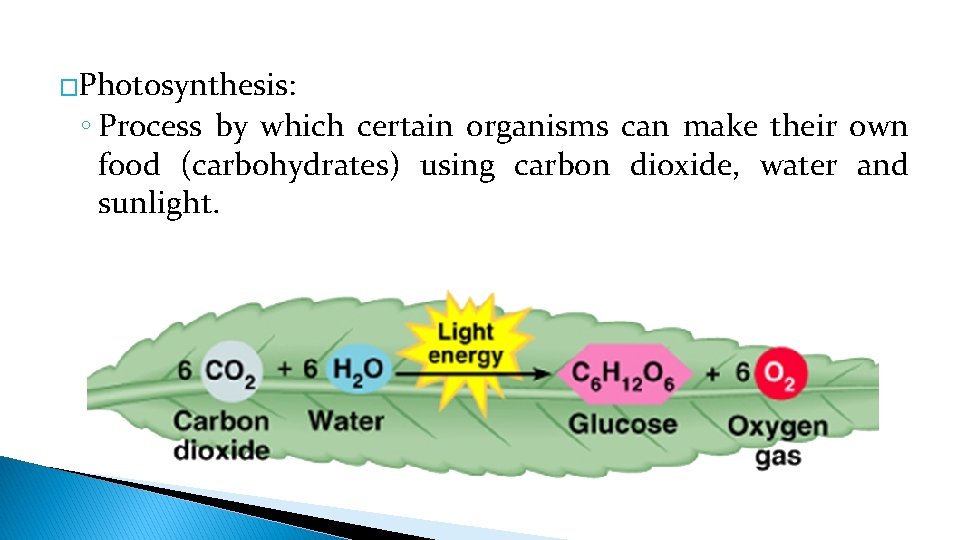
�Photosynthesis: ◦ Process by which certain organisms can make their own food (carbohydrates) using carbon dioxide, water and sunlight.
�Environmental parameters can affect the rate of photosynthesis. �An increase or decrease in: �Reactant; �Availability of light energy; or �Temperature ◦ Leads to an increase or decrease in the rate of photosynthesis (to an extent).
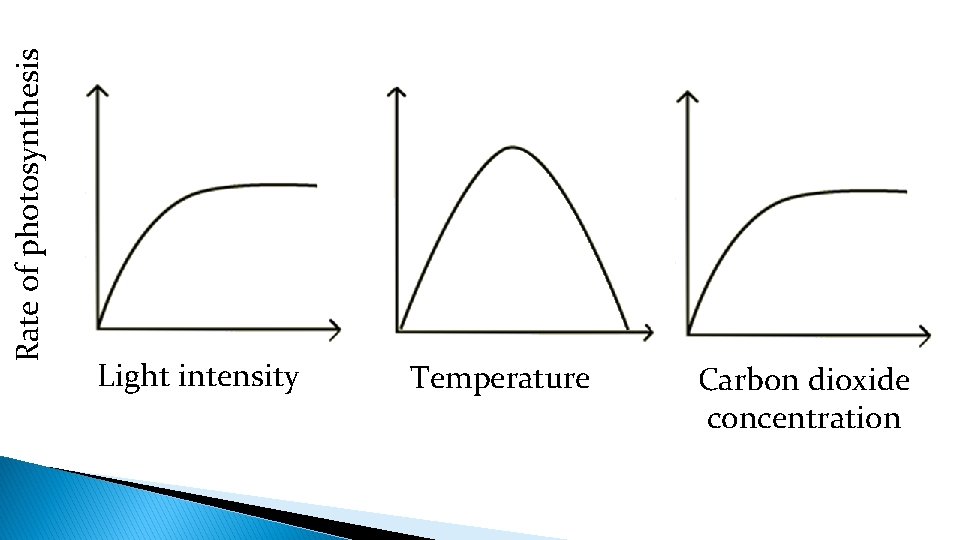
Rate of photosynthesis Light intensity Temperature Carbon dioxide concentration

◦ Site allows you to change three environmental factors that affect the rate of photosynthesis: �Temperature. �Carbon dioxide concentration. �Light intensity. http: //www. kscience. co. uk/animations/photolab. swf
◦ Rate of photosynthesis is shown by production of bubbles, representing production of oxygen gas (product of photosynthesis). �More bubbles = higher rate of photosynthesis. ◦ Run all 18 combinations of environmental parameters. �Use timer function included in animation, count # bubbles produced during 30 seconds, record result. �Complete column headed “rate of photosynthesis # bubbles/min” by multiplying the value from the previous column by 2.
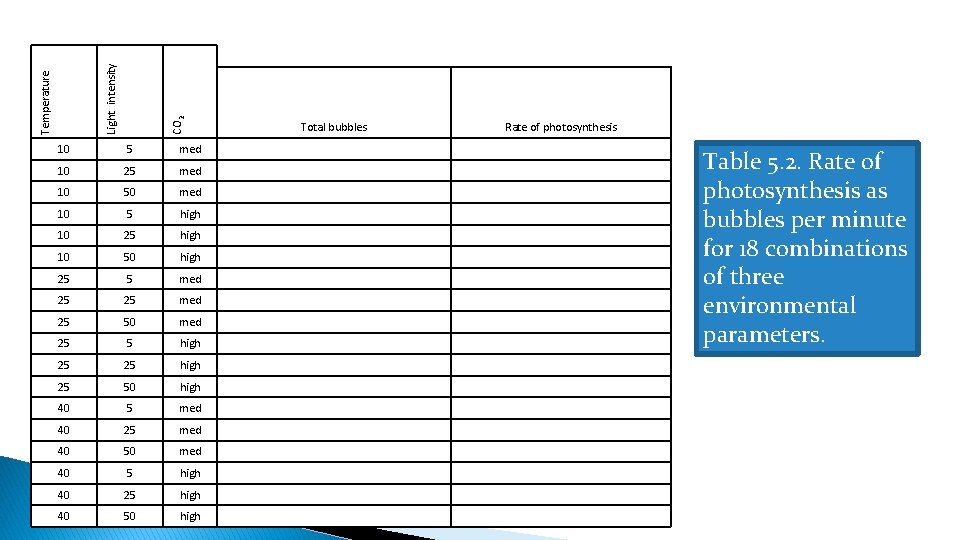
CO 2 Light intensity Temperature 10 5 med 10 25 med 10 50 med 10 5 high 10 25 high 10 50 high 25 5 med 25 25 med 25 50 med 25 5 high 25 25 high 25 50 high 40 5 med 40 25 med 40 50 med 40 5 high 40 25 high 40 50 high Total bubbles Rate of photosynthesis Table 5. 2. Rate of photosynthesis as bubbles per minute for 18 combinations of three environmental parameters.
38 36 34 32 # bubbles per minute 30 28 Figure 5. 1. Rate of photosynthesis (bubbles per minute) at light intensity 5, medium and high concentrations of CO 2 and three temperatures. 26 24 22 20 18 16 14 12 10 8 6 4 2 0 Median CO 2 High CO 2 10 °C Medium CO 2 25 °C High CO 2 Medium CO 2 40 °C High CO 2
38 36 34 32 # bubbles per minute 30 28 Figure 5. 2. Rate of photosynthesis (bubbles per minute) at light intensity 25, medium and high concentrations of CO 2 and three temperatures. 26 24 22 20 18 16 14 12 10 8 6 4 2 0 Median CO 2 High CO 2 10 °C Medium CO 2 25 °C High CO 2 Medium CO 2 40 °C High CO 2
38 36 34 32 # bubbles per minute 30 28 Figure 5. 3. Rate of photosynthesis (bubbles per minute) at light intensity 50, medium and high concentrations of CO 2 and three temperatures. 26 24 22 20 18 16 14 12 10 8 6 4 2 0 Median CO 2 High CO 2 10 °C Medium CO 2 25 °C High CO 2 Medium CO 2 40 °C High CO 2

What’s Due �Weekly Data Sheets (Due Today): • P. 83 , 93 103. �Weekly Write-Ups (Due Next Class): • P. 87 -88, 97 -98, 107 -110. P. 83: OK to hand in hard copy Power. Point at https: //eeltown. org/evpp-110 -spring-2018/
- Slides: 38