Evolution of Oxygen in the Atmosphere Early reactions

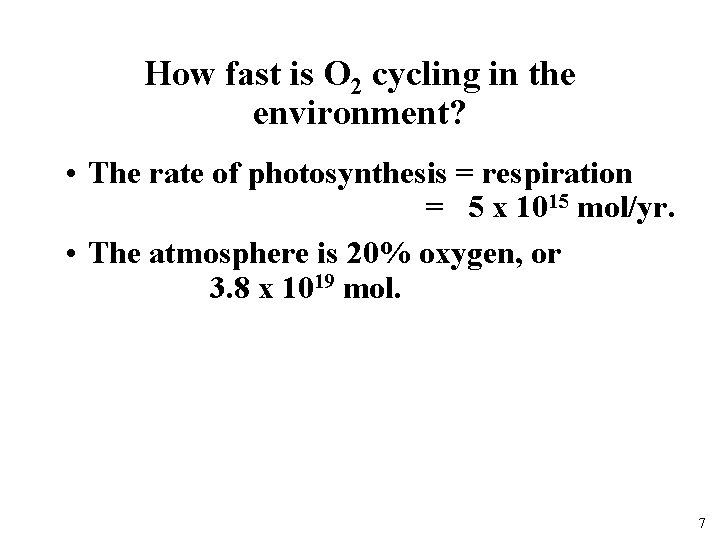



- Slides: 14

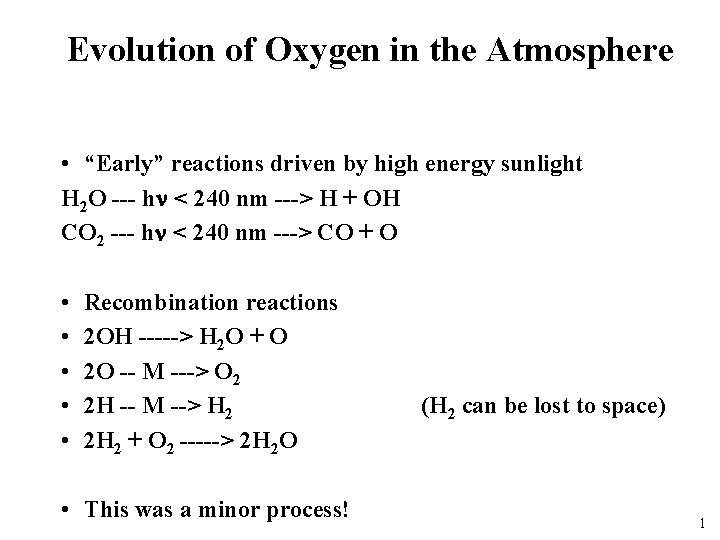
Evolution of Oxygen in the Atmosphere • “Early” reactions driven by high energy sunlight H 2 O --- hn < 240 nm ---> H + OH CO 2 --- hn < 240 nm ---> CO + O • • • Recombination reactions 2 OH -----> H 2 O + O 2 O -- M ---> O 2 2 H -- M --> H 2 2 H 2 + O 2 -----> 2 H 2 O • This was a minor process! (H 2 can be lost to space) 1

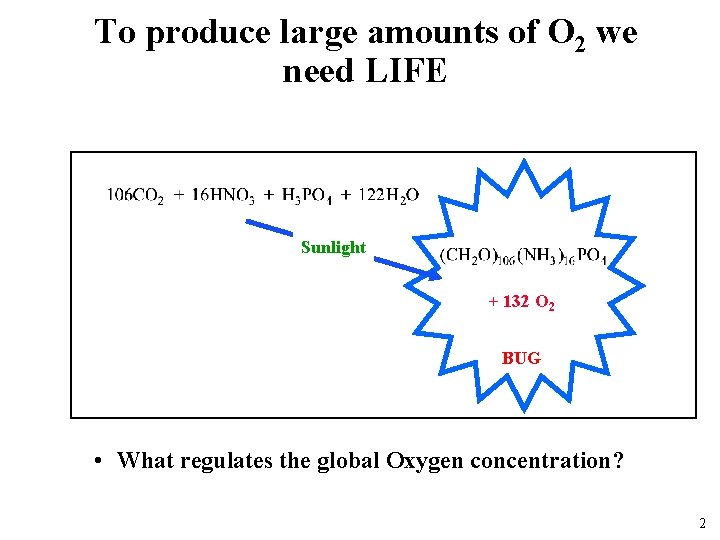
To produce large amounts of O 2 we need LIFE Sunlight + 132 O 2 BUG • What regulates the global Oxygen concentration? 2

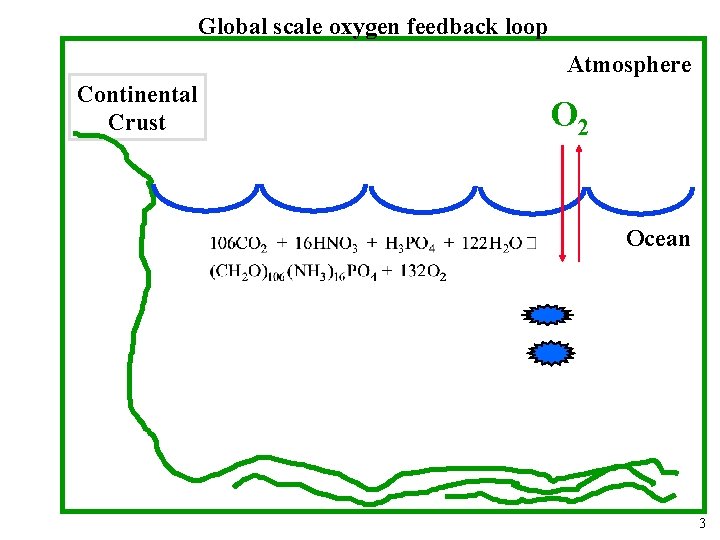
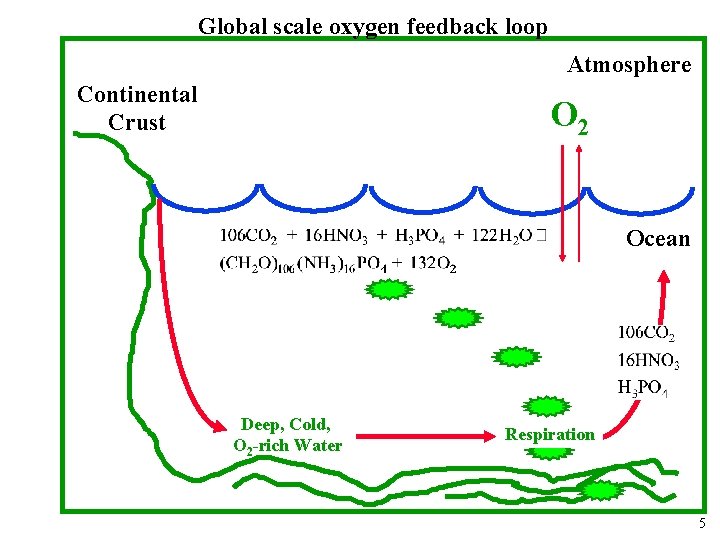
Global scale oxygen feedback loop Atmosphere Continental Crust O 2 Ocean 3

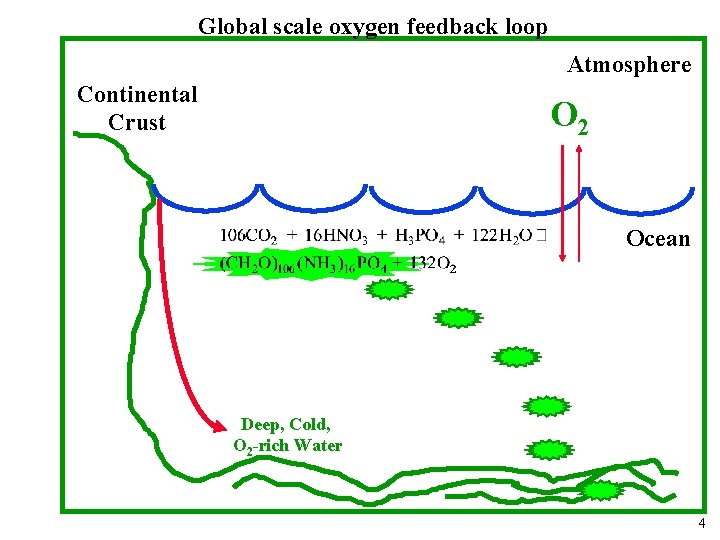
Global scale oxygen feedback loop Atmosphere Continental Crust O 2 Ocean Deep, Cold, O 2 -rich Water 4

Global scale oxygen feedback loop Atmosphere Continental Crust O 2 Ocean Deep, Cold, O 2 -rich Water Respiration 5

Feedback on Atmospheric O 2 • “Correct” Atmospheric Oxygen Photosynthesis = Respiration • Too Little Atmospheric Oxygen Photosynthesis >> Respiration • Too Much Atmospheric Oxygen Photosynthesis << Respiration 6
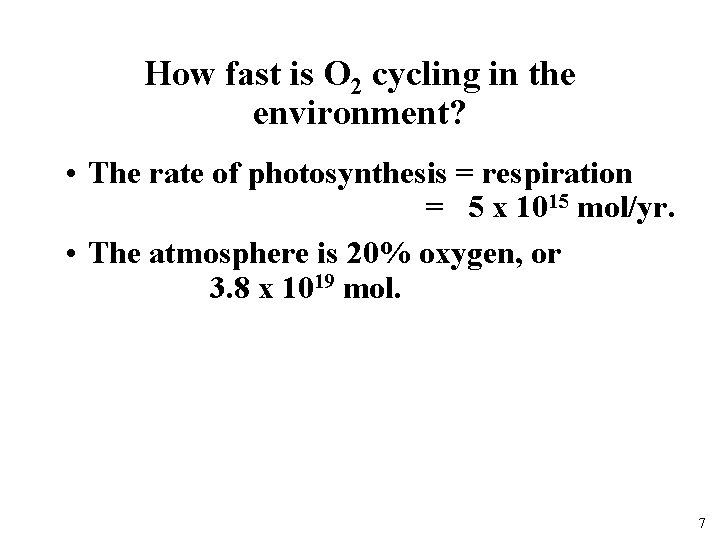
How fast is O 2 cycling in the environment? • The rate of photosynthesis = respiration = 5 x 1015 mol/yr. • The atmosphere is 20% oxygen, or 3. 8 x 1019 mol. 7

How fast is O 2 cycling in the environment? • The rate of photosynthesis = respiration = 5 x 1015 mol/yr. • The atmosphere is 20% oxygen, or 3. 8 x 1019 mol. • Residence time for oxygen = 7600 years • What does this mean? ? Oxygen is well mixed in the atmosphere! 8

Nitrogen in the atmosphere • Nitrogen was outgassed early in Earth formation and constitutes 80% of the atmosphere. (1. 4 x 1020 moles) • Two major sinks: – N 2(g) + O 2(g) ----- >T ----> 2 NO(g) – N 2(g) + 3 H 2(g) ---- >T, catalyst ---> 2 NH 3(g) – Total flux = 4. 6 x 1012 moles/year • Residence time = 3 x 107 years 9

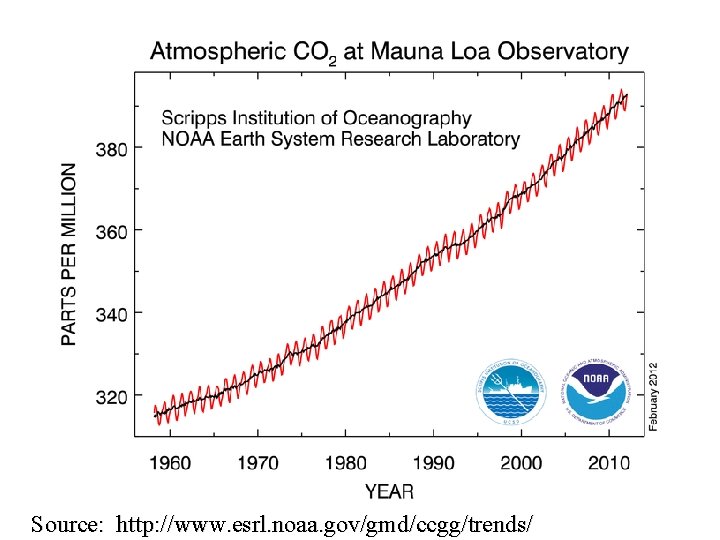
Source: http: //www. esrl. noaa. gov/gmd/ccgg/trends/ 10

CO 2 � Mass in the atmosphere 700 Gt � Annual flux to the atmosphere is about 150 Gt � Residence time = 5 years � Does this make sense with observed CO 2? 11

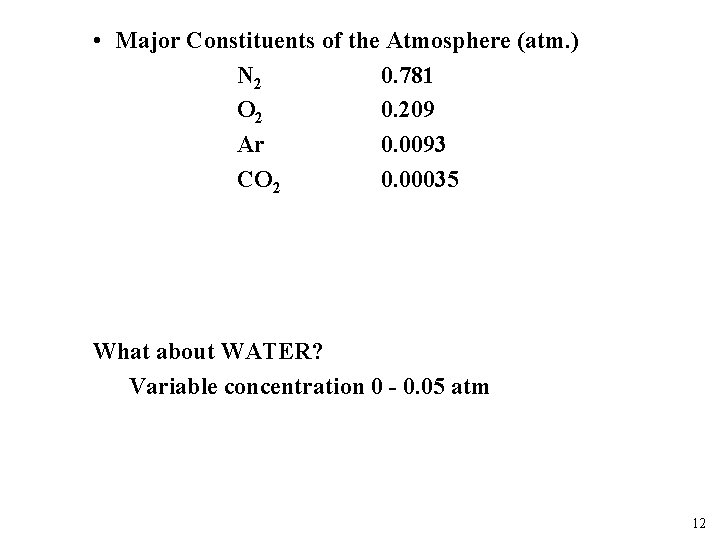
• Major Constituents of the Atmosphere (atm. ) N 2 0. 781 O 2 0. 209 Ar 0. 0093 CO 2 0. 00035 What about WATER? Variable concentration 0 - 0. 05 atm 12

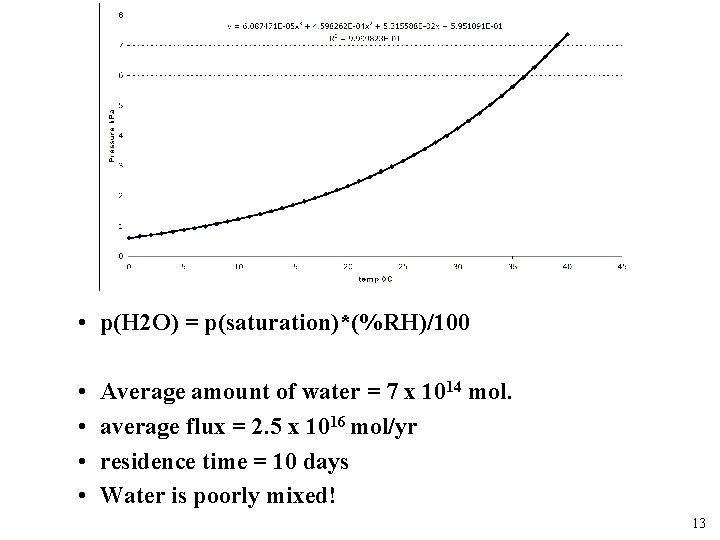
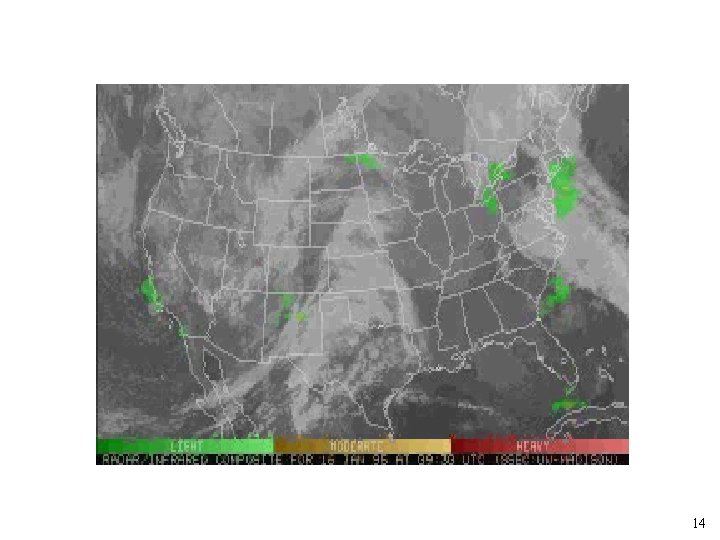
Temperature --> (o. C) • p(H 2 O) = p(saturation)*(%RH)/100 • • Average amount of water = 7 x 1014 mol. average flux = 2. 5 x 1016 mol/yr residence time = 10 days Water is poorly mixed! 13

14