Evolution of Neoplasia The Uterine Cervix As a

- Slides: 45

Evolution of Neoplasia The Uterine Cervix As a Model Raj C. Dash, MD Duke University Medical Center Durham, North Carolina

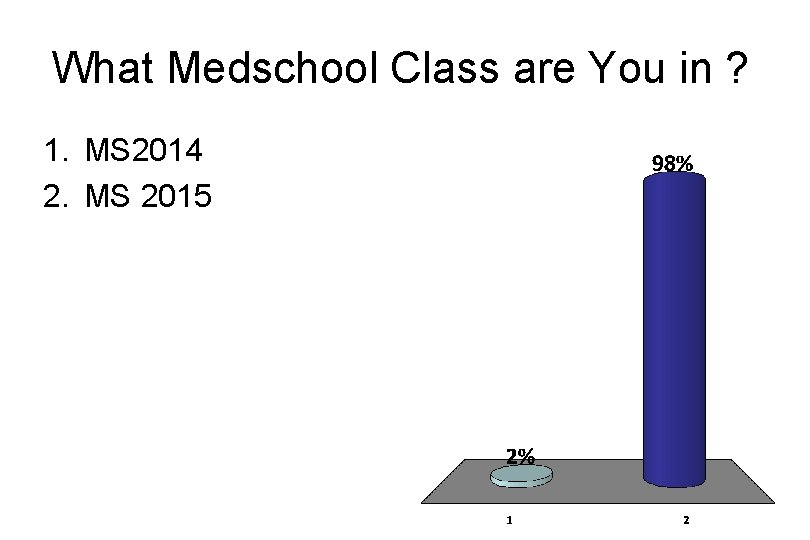
What Medschool Class are You in ? 1. MS 2014 2. MS 2015

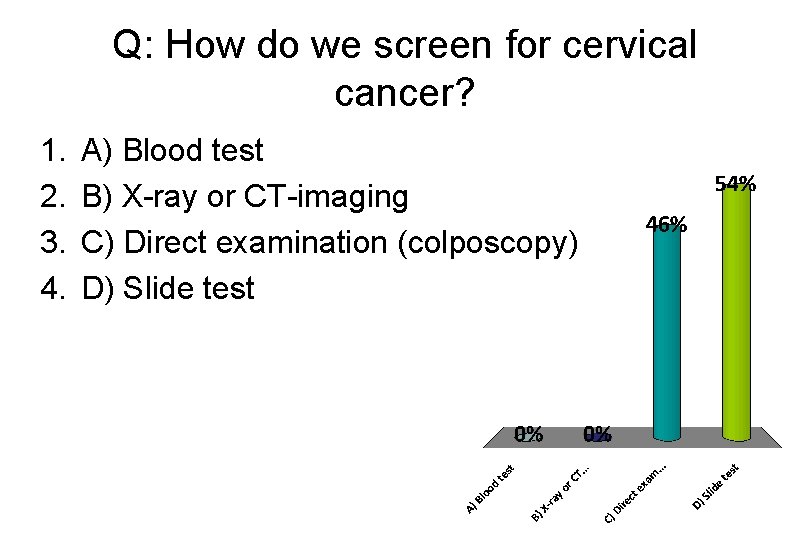
Q: How do we screen for cervical cancer? 1. 2. 3. 4. A) Blood test B) X-ray or CT-imaging C) Direct examination (colposcopy) D) Slide test

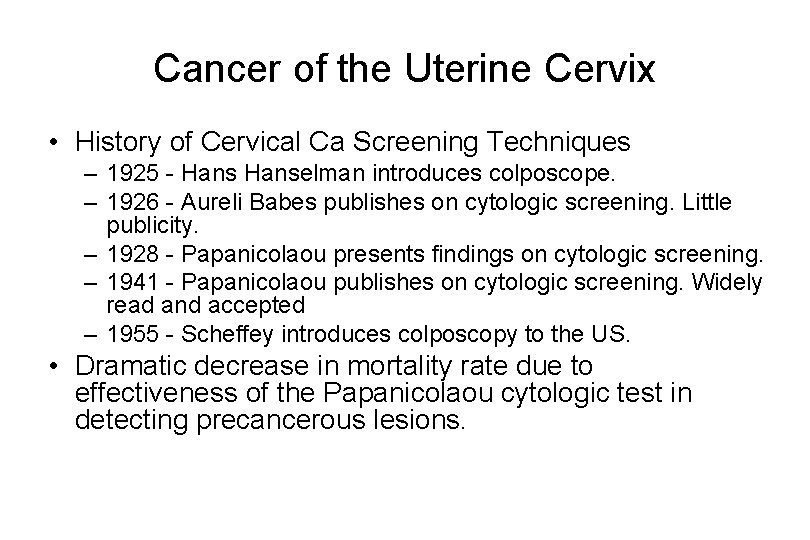
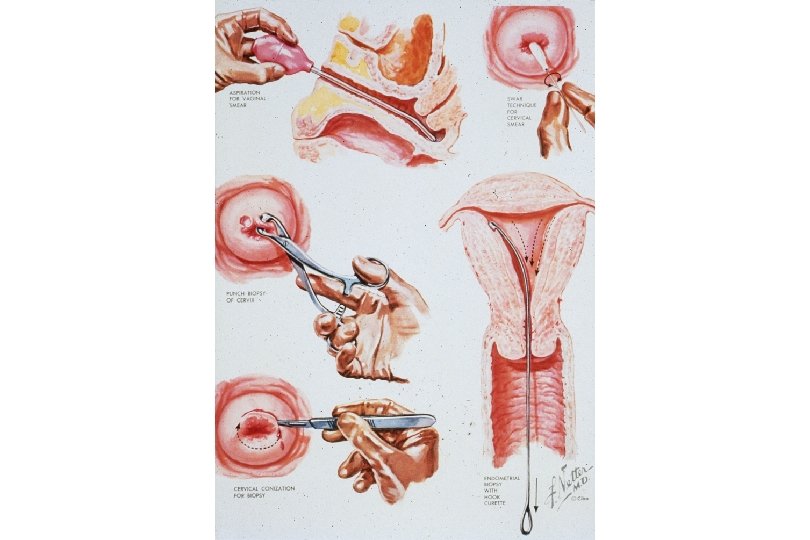
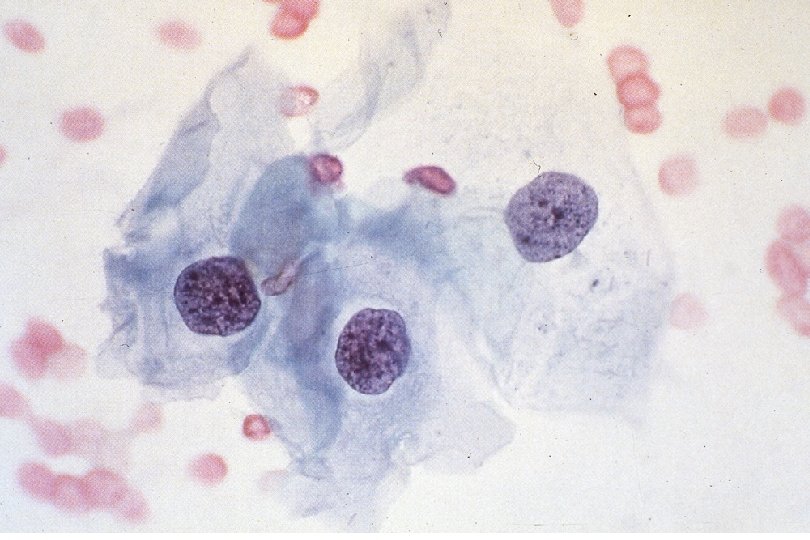
Cancer of the Uterine Cervix • History of Cervical Ca Screening Techniques – 1925 - Hanselman introduces colposcope. – 1926 - Aureli Babes publishes on cytologic screening. Little publicity. – 1928 - Papanicolaou presents findings on cytologic screening. – 1941 - Papanicolaou publishes on cytologic screening. Widely read and accepted – 1955 - Scheffey introduces colposcopy to the US. • Dramatic decrease in mortality rate due to effectiveness of the Papanicolaou cytologic test in detecting precancerous lesions.

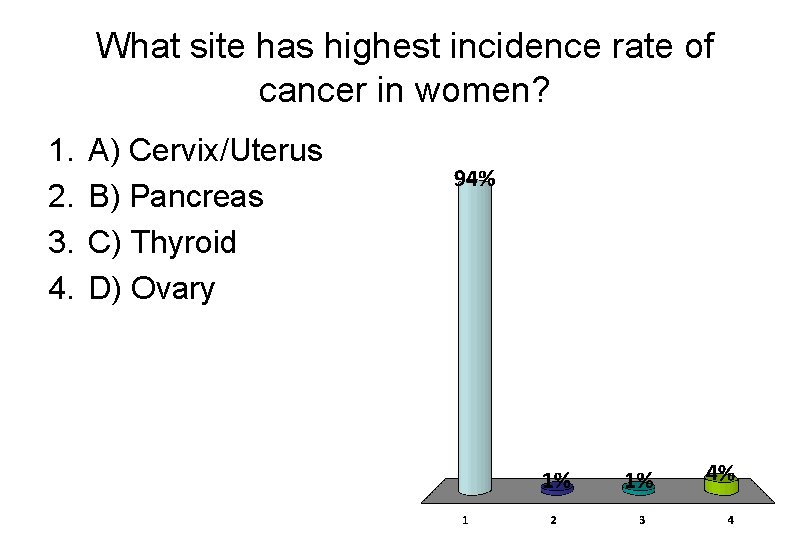
What site has highest incidence rate of cancer in women? 1. 2. 3. 4. A) Cervix/Uterus B) Pancreas C) Thyroid D) Ovary

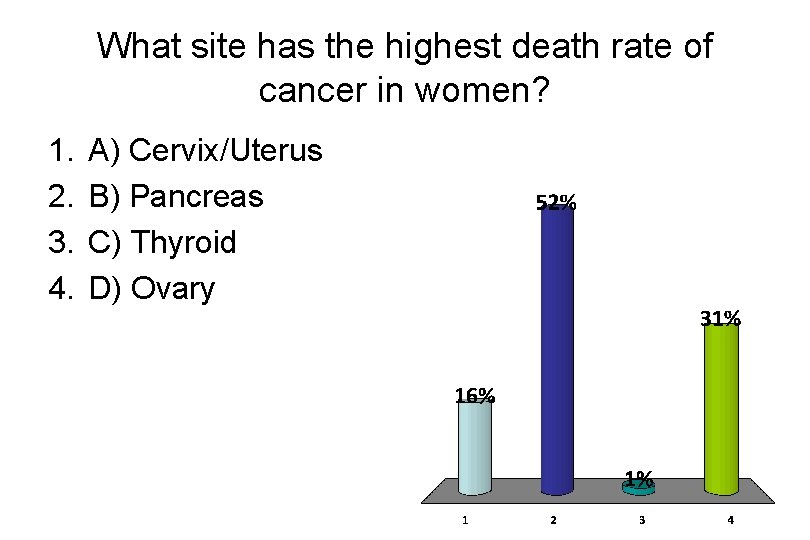
What site has the highest death rate of cancer in women? 1. 2. 3. 4. A) Cervix/Uterus B) Pancreas C) Thyroid D) Ovary

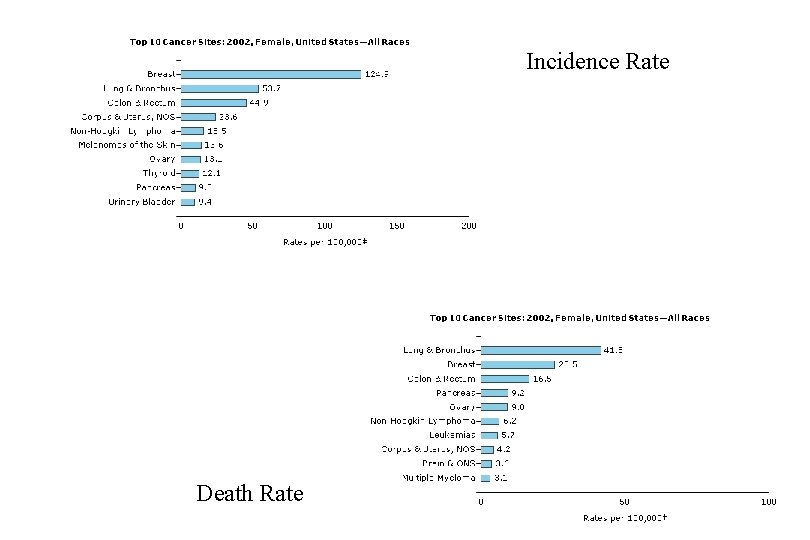
Incidence Rate Death Rate

Cervical Cancer Epidemiology • • 20, 000 new cases per year 7, 600 deaths per year 3. 5% of all female deaths per year 50 million women undergo Pap testing in the U. S. / year. • 3. 5 million (7%) are diagnosed with a cytological abnormality requiring additional follow-up or evaluation. JAMA. 2002; 287: 2120 -2129.

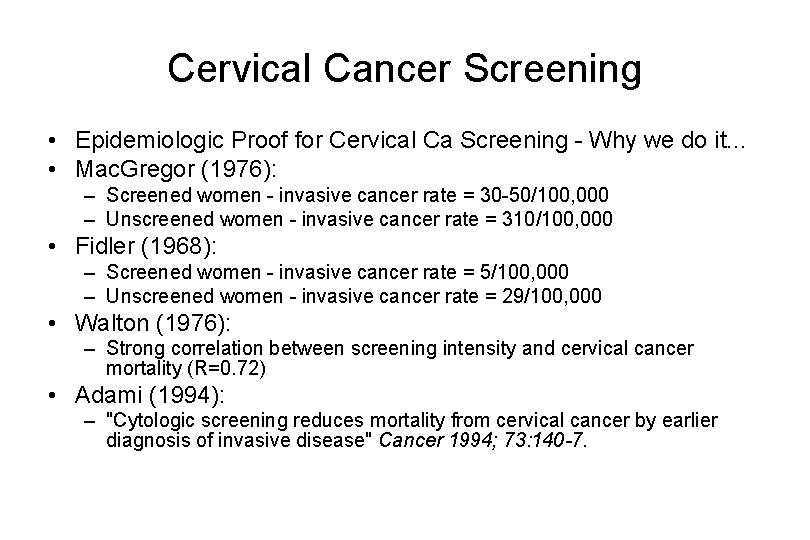
Cervical Cancer Screening • Epidemiologic Proof for Cervical Ca Screening - Why we do it. . . • Mac. Gregor (1976): – Screened women - invasive cancer rate = 30 -50/100, 000 – Unscreened women - invasive cancer rate = 310/100, 000 • Fidler (1968): – Screened women - invasive cancer rate = 5/100, 000 – Unscreened women - invasive cancer rate = 29/100, 000 • Walton (1976): – Strong correlation between screening intensity and cervical cancer mortality (R=0. 72) • Adami (1994): – "Cytologic screening reduces mortality from cervical cancer by earlier diagnosis of invasive disease" Cancer 1994; 73: 140 -7.



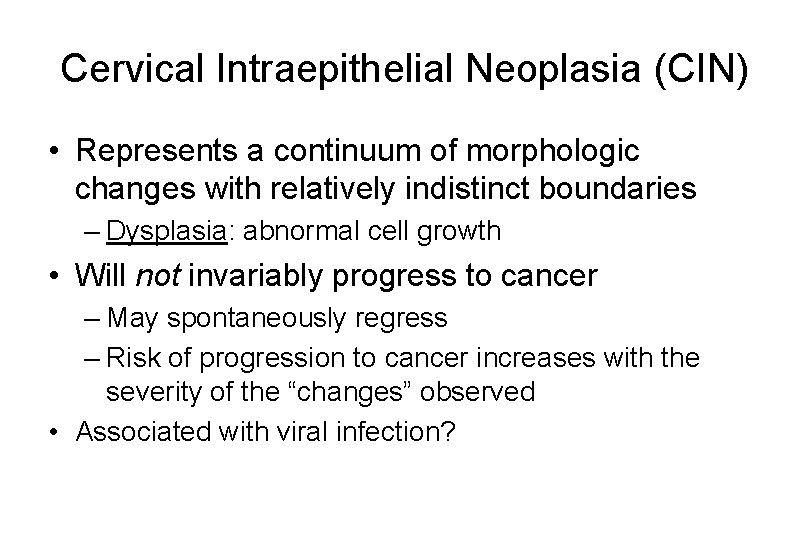
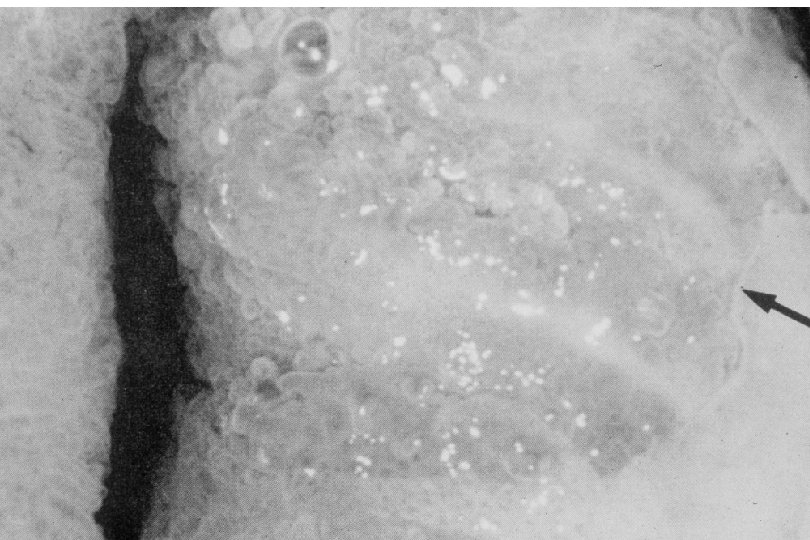
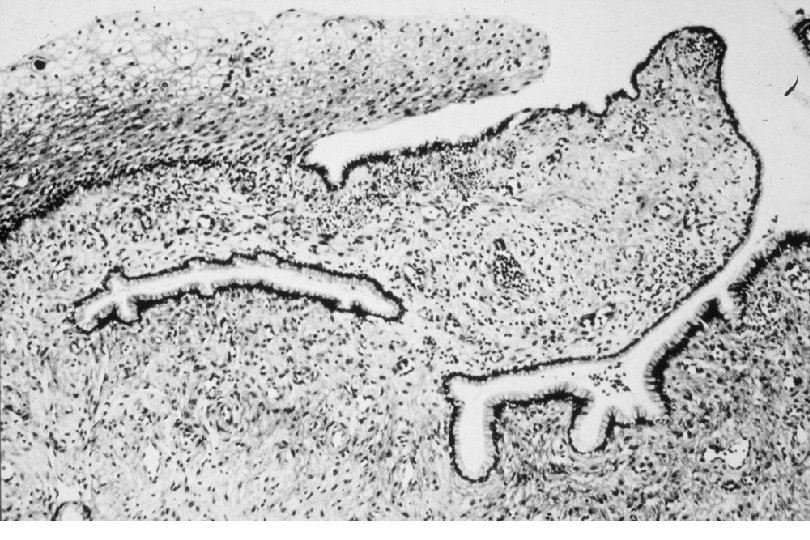
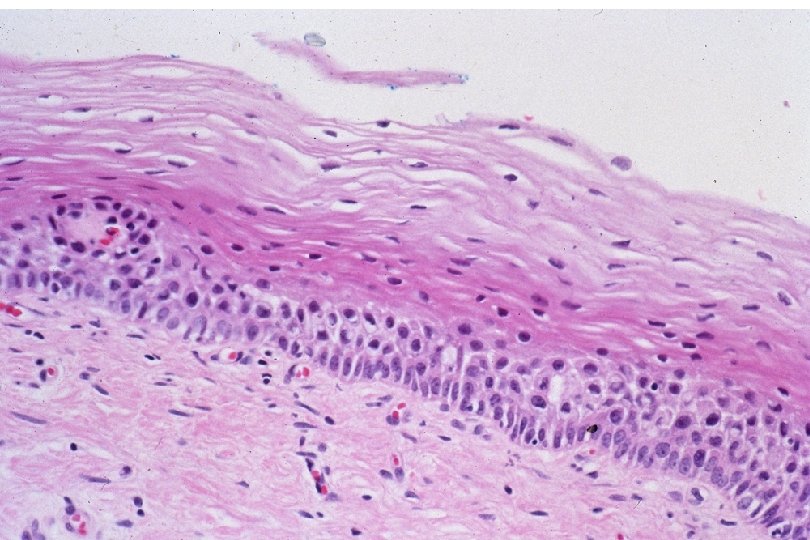
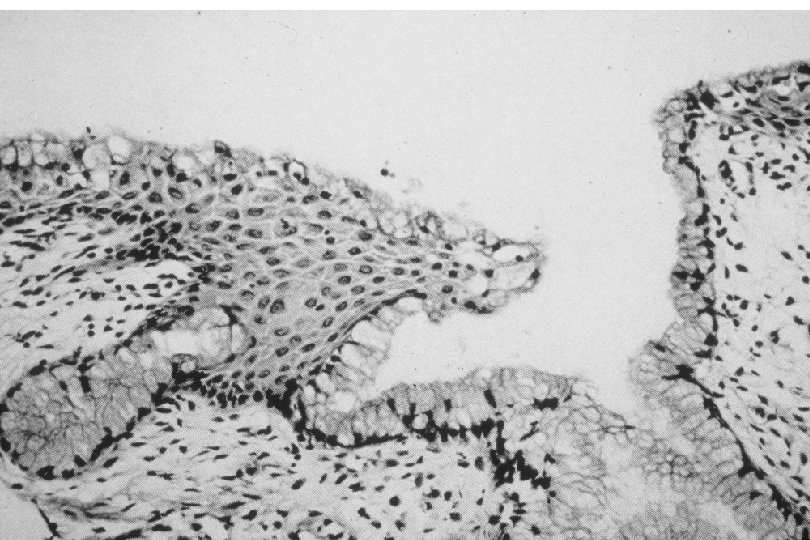
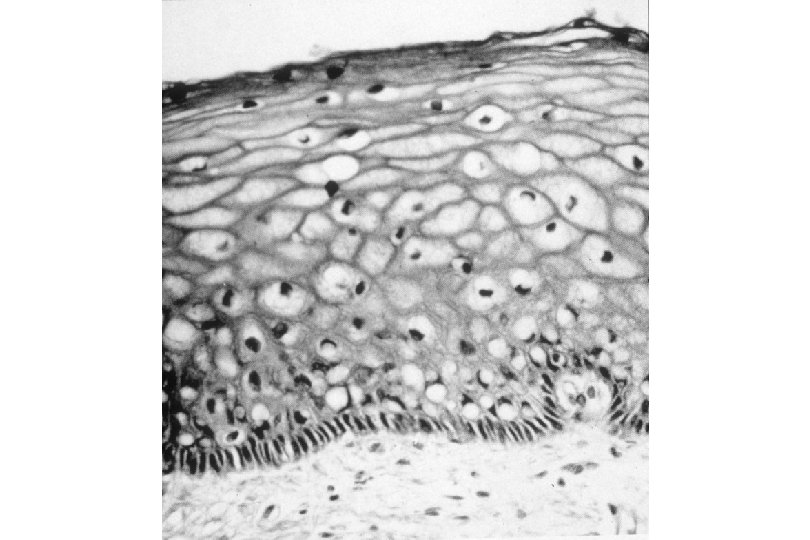
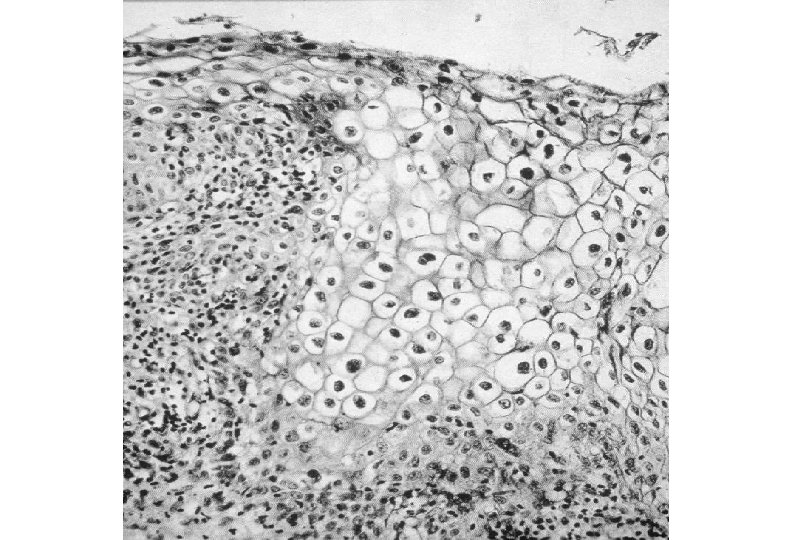
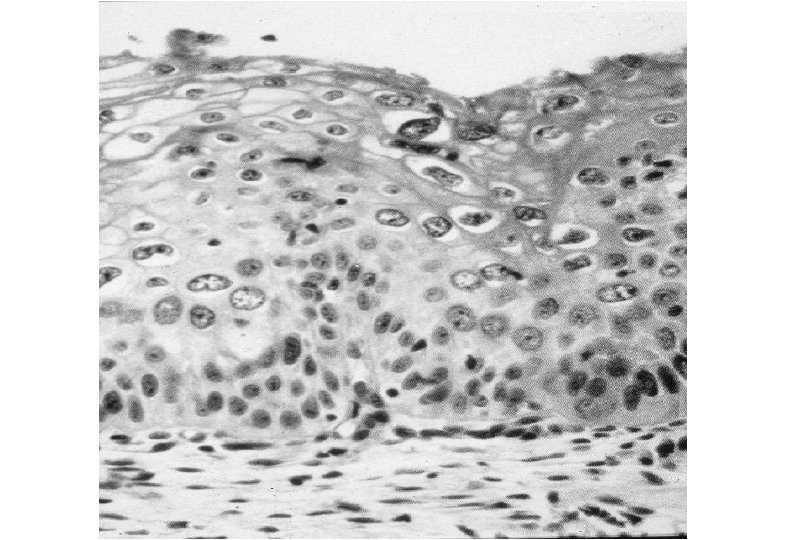
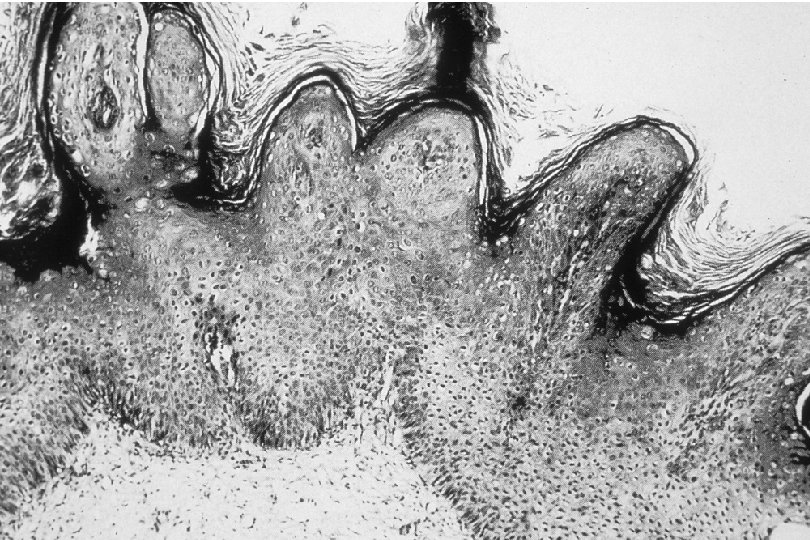
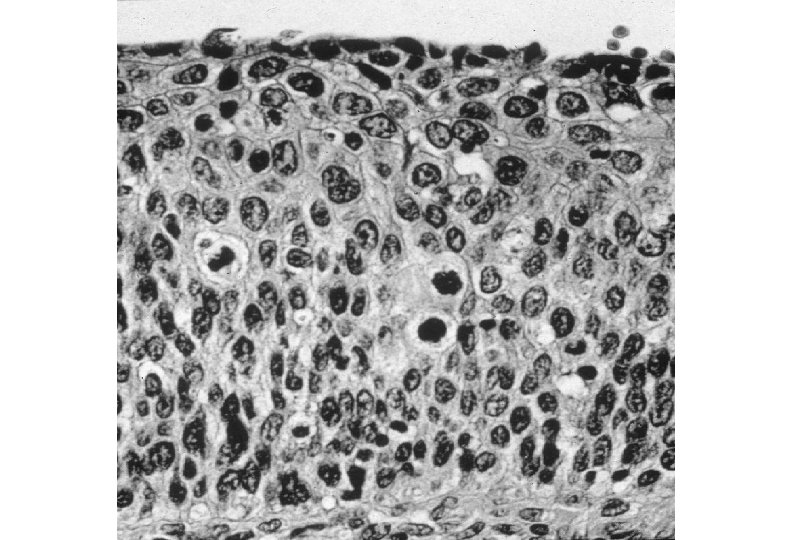
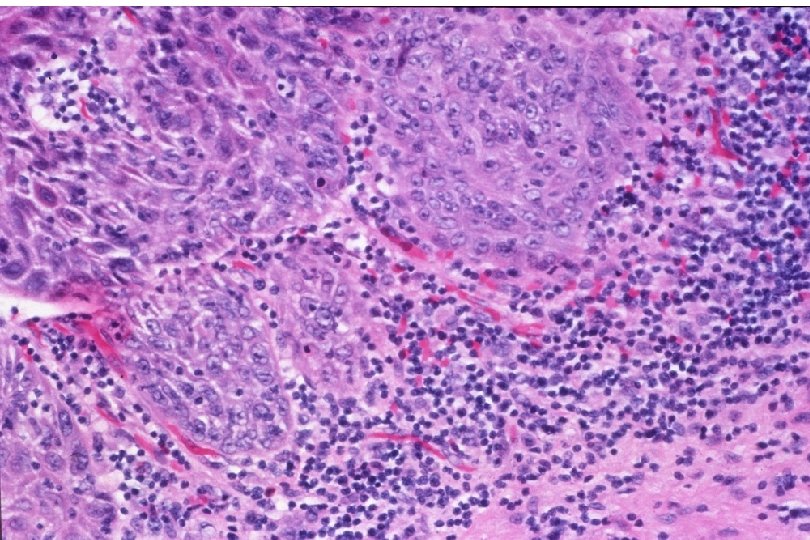
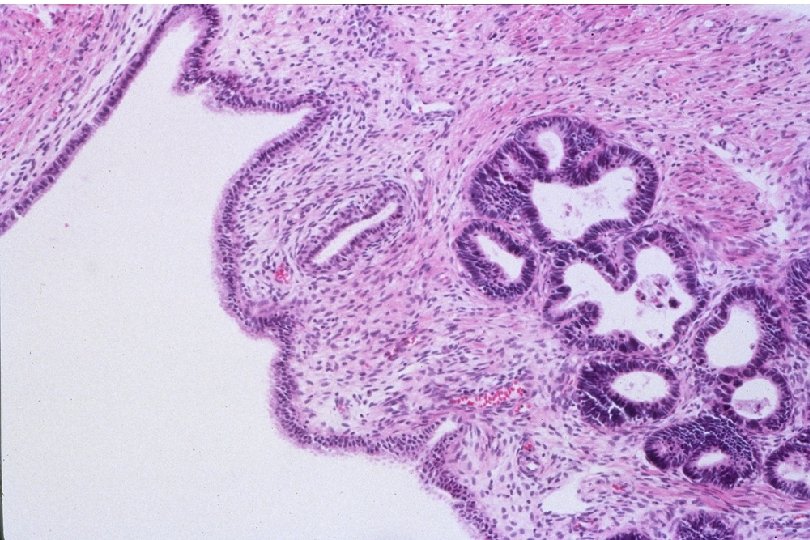
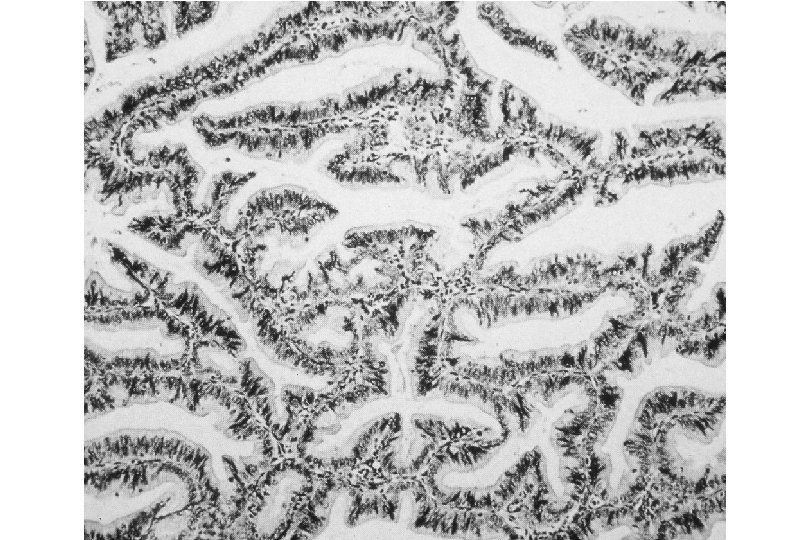
Cervical Intraepithelial Neoplasia (CIN) • Represents a continuum of morphologic changes with relatively indistinct boundaries – Dysplasia: abnormal cell growth • Will not invariably progress to cancer – May spontaneously regress – Risk of progression to cancer increases with the severity of the “changes” observed • Associated with viral infection?

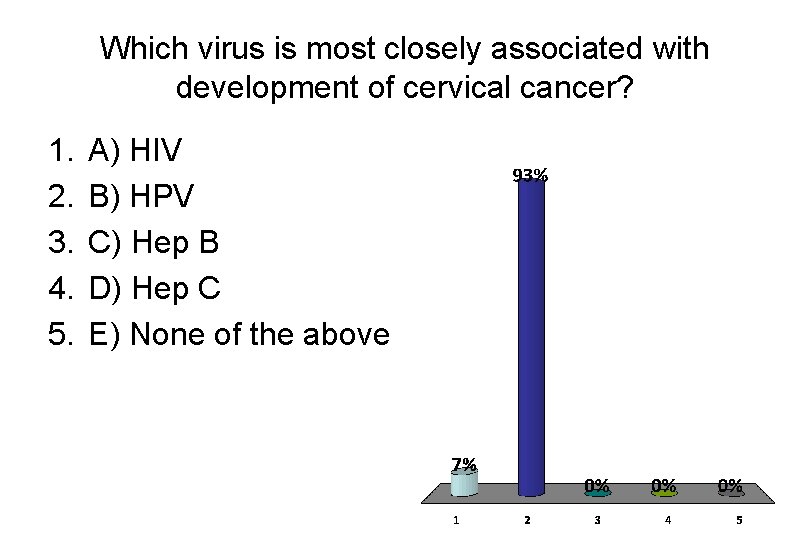
Which virus is most closely associated with development of cervical cancer? 1. 2. 3. 4. 5. A) HIV B) HPV C) Hep B D) Hep C E) None of the above

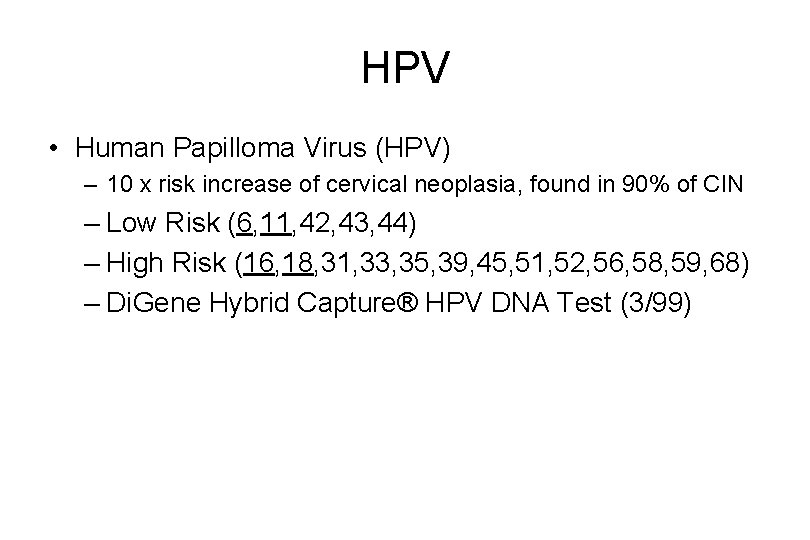
HPV • Human Papilloma Virus (HPV) – 10 x risk increase of cervical neoplasia, found in 90% of CIN – Low Risk (6, 11, 42, 43, 44) – High Risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) – Di. Gene Hybrid Capture® HPV DNA Test (3/99)

Nomenclature • Tumors are composed of proliferating neoplastic cells (clones) and supportive stroma of connective tissue and blood vessels. • Tumors are named according to their neoplastic component.

Malignancy Nomenclature • Carcinoma: malignant neoplasia arising from epithelial tissue. • Sarcoma: malignant neoplasia arising from mesenchymal (connective) tissue. • Lymphoma: malignant neoplasm arising from lymphoid tissue.

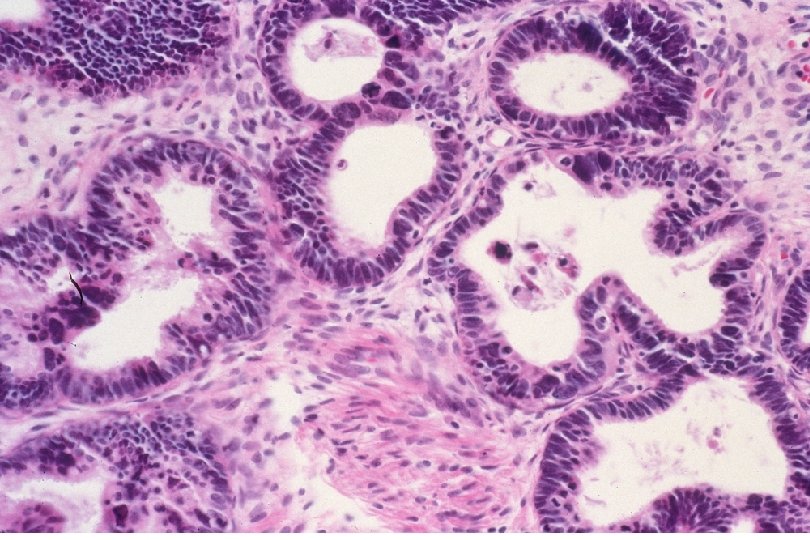
Nomenclature • Carcinoma – Squamous cell carcinoma: derived from squamous epithelium. – Adenocarcinoma: derived from glandular epithelium.

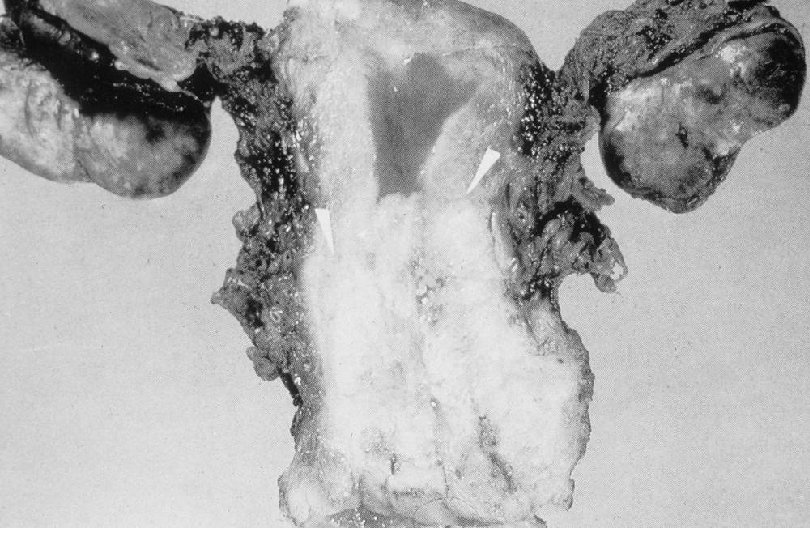
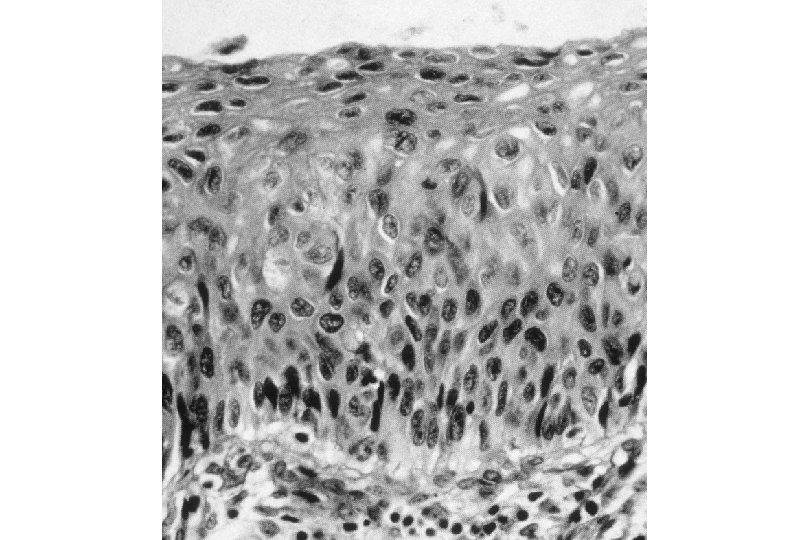
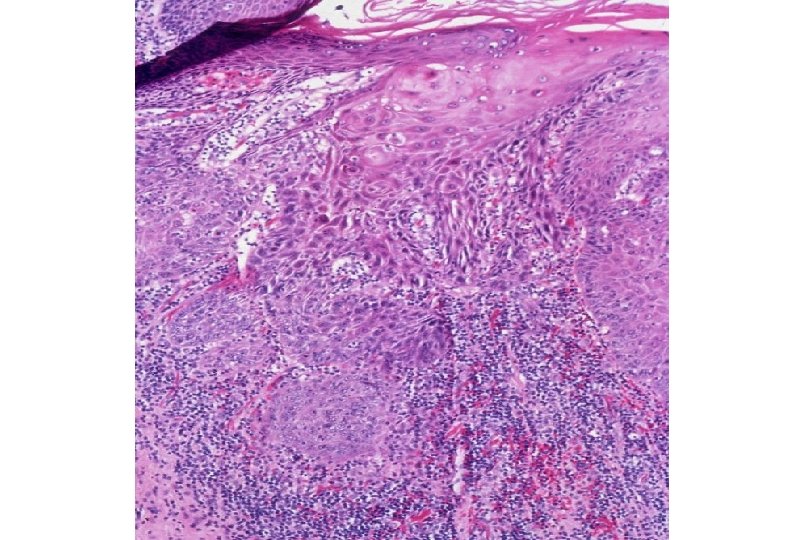
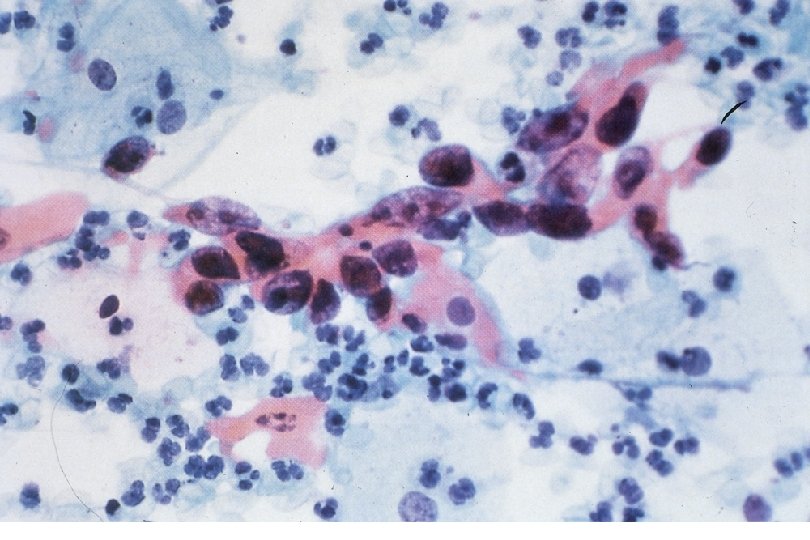
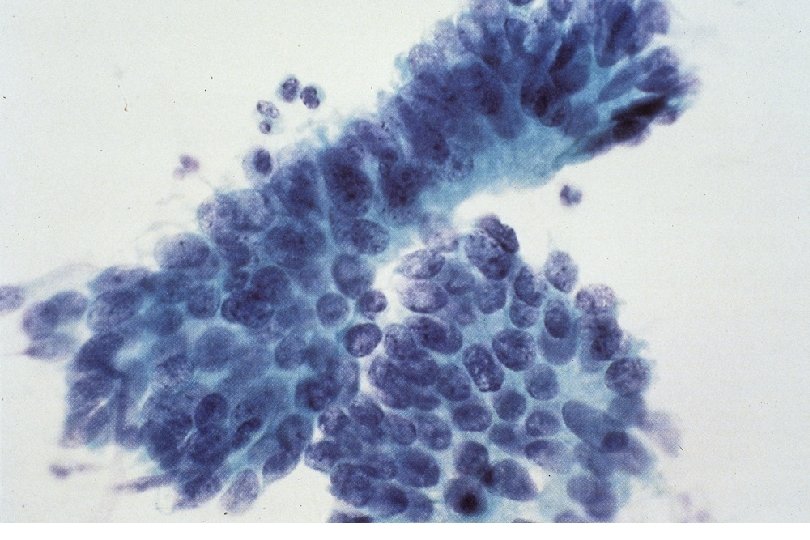
Malignant Characteristics • Rapid, Autonomous Growth – “Anaplasia” (uncontrolled growth bearing little similarity to cell of origin, implies progression beyond severe dysplasia, beyond well-differentiated malignancy; often associated with “poor differentiation”) • Locally invasive • Potential for metastatic spread – Lymphovascular invasion

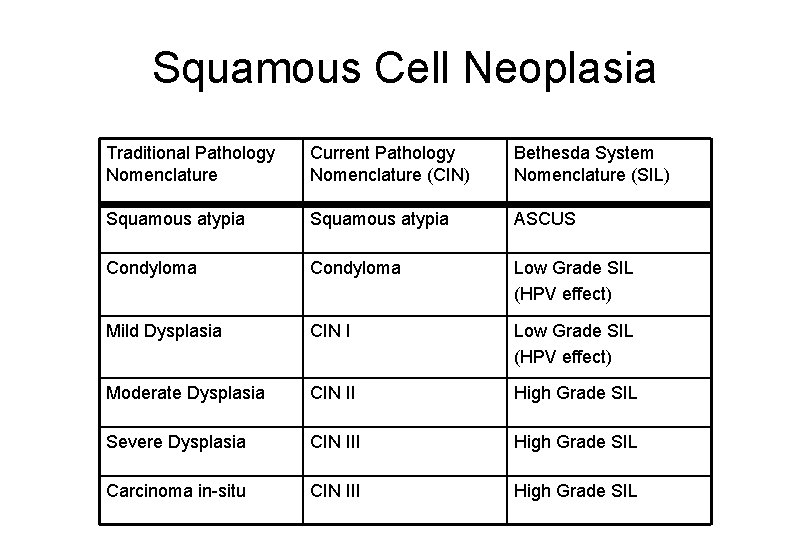
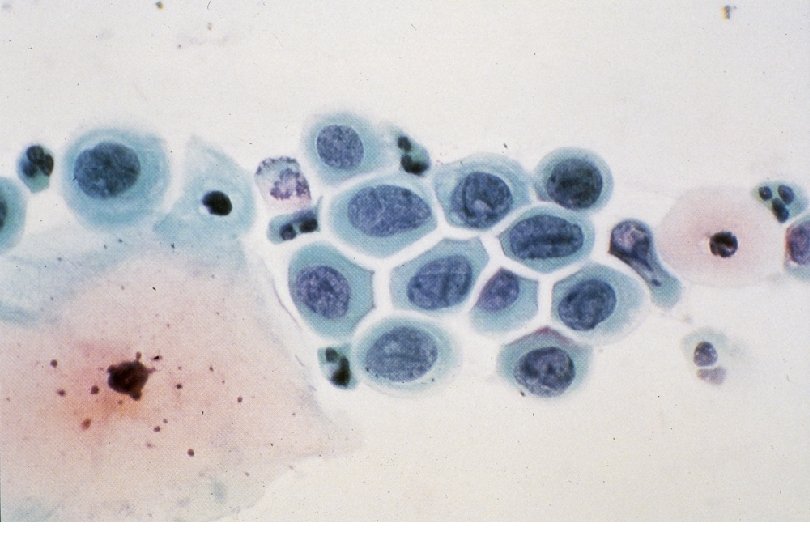
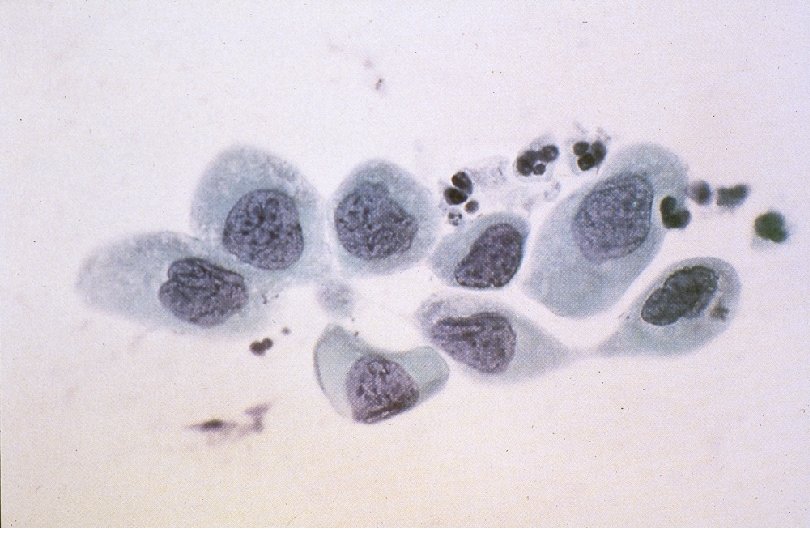
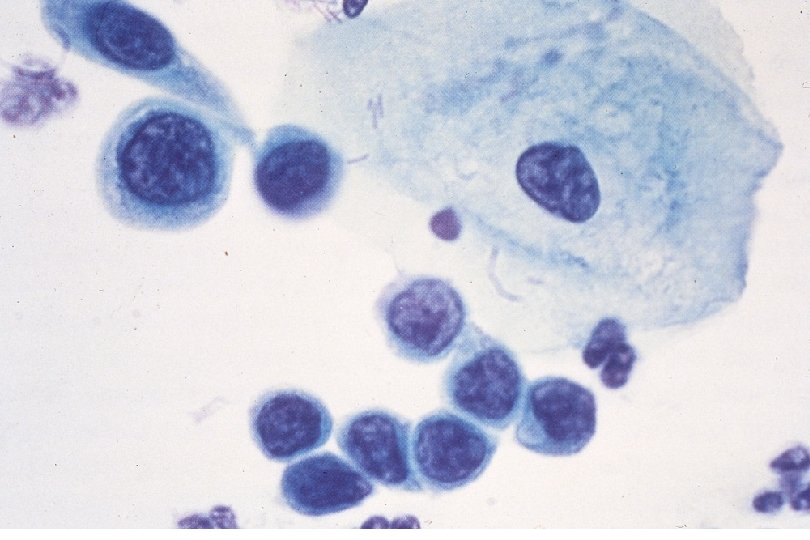
Squamous Cell Neoplasia Traditional Pathology Nomenclature Current Pathology Nomenclature (CIN) Bethesda System Nomenclature (SIL) Squamous atypia ASCUS Condyloma Low Grade SIL (HPV effect) Mild Dysplasia CIN I Low Grade SIL (HPV effect) Moderate Dysplasia CIN II High Grade SIL Severe Dysplasia CIN III High Grade SIL Carcinoma in-situ CIN III High Grade SIL

Quick Quiz: Severe Dysplasia matches… 1. 2. 3. 4. 5. A) LSIL / CIN I B) LSIL / CIN II C) LSIL / CIN III D) HSIL / CIN II E) HSIL / CIN III

























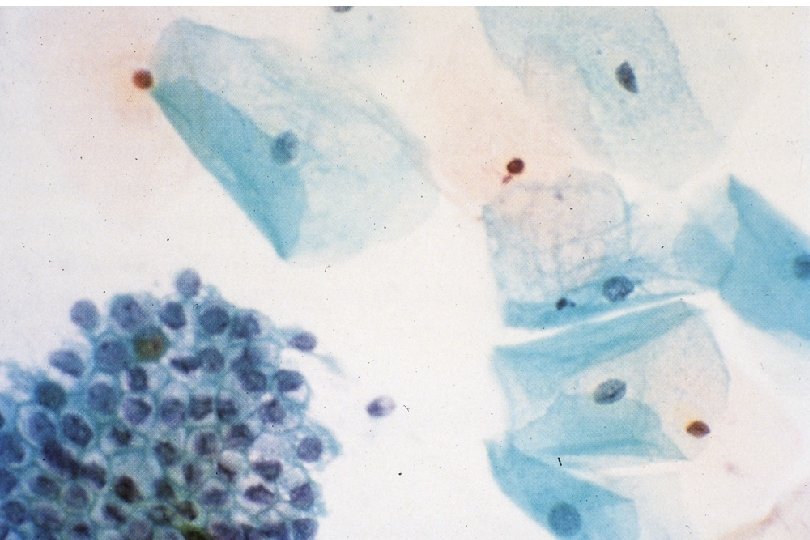
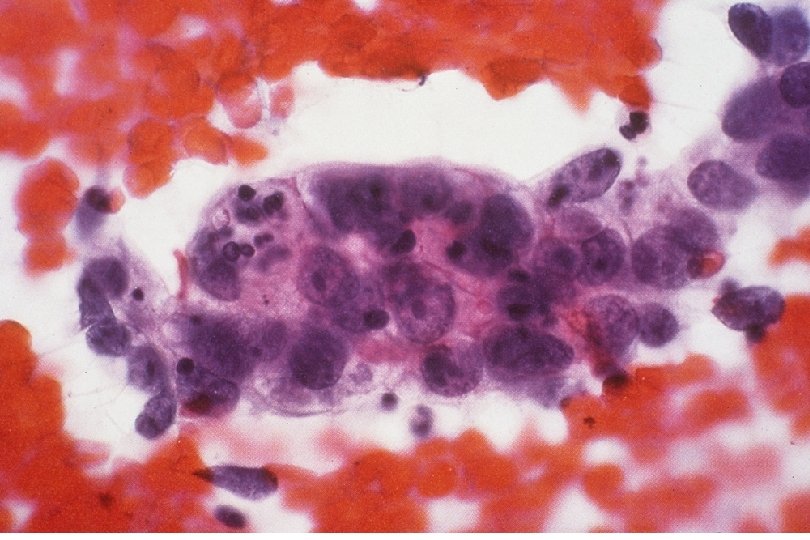
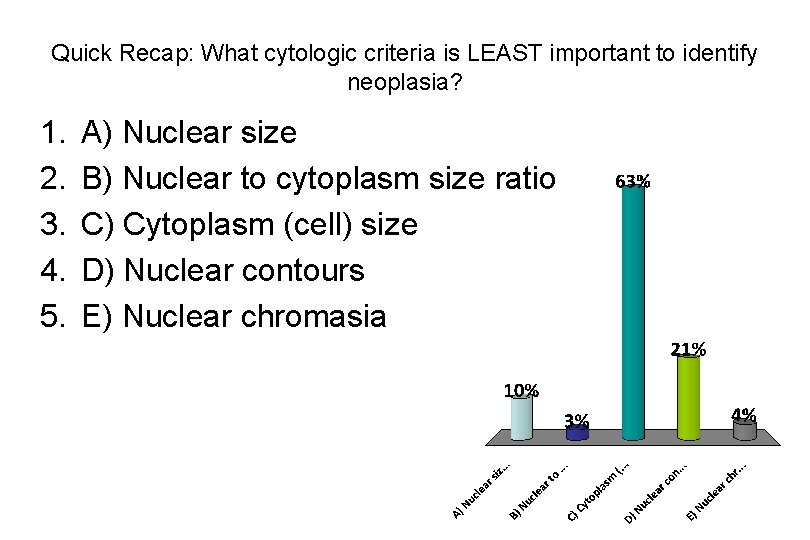
Quick Recap: What cytologic criteria is LEAST important to identify neoplasia? 1. 2. 3. 4. 5. A) Nuclear size B) Nuclear to cytoplasm size ratio C) Cytoplasm (cell) size D) Nuclear contours E) Nuclear chromasia