EVITHERM WORK 2004 FOOD A RAEMY Nestl Research








![Evitherm 2004: Specific heat = General formula is: Q=mc T [Q] = J or Evitherm 2004: Specific heat = General formula is: Q=mc T [Q] = J or](https://slidetodoc.com/presentation_image_h2/4d15e5c0f4e831fbecc667dfcaf70b1c/image-12.jpg)
![Evitherm-2004: Specific heat Theoretical calculation Specific heat of food constituents [1] - For water: Evitherm-2004: Specific heat Theoretical calculation Specific heat of food constituents [1] - For water:](https://slidetodoc.com/presentation_image_h2/4d15e5c0f4e831fbecc667dfcaf70b1c/image-14.jpg)

![Evitherm 2004: Specific heat = Literature on specific heat of foods [1] N. N. Evitherm 2004: Specific heat = Literature on specific heat of foods [1] N. N.](https://slidetodoc.com/presentation_image_h2/4d15e5c0f4e831fbecc667dfcaf70b1c/image-20.jpg)

- Slides: 21

EVITHERM WORK 2004 : FOOD A. RAEMY Nestlé Research Centre Nestec LTD CH- 1000 Lausanne 26 Switzerland According to the request of Joachim Fischer, PTB, Berlin 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: data main requirements (1) = Specific heats of foods and some non food materials (mainly packaging and process materials such as stainless steel, copper, polymers, …) = Glass transition temperatures which are abrupt changes in specific heats = Thermal conductivity = Basics of thermometry 2 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: data main requirements (2) = Phase transition (melting, …) temperatures = Reaction (oxidation, Maillard, …) onset temperatures = Transition and reaction enthalpy values = Enthalpies normed at – 60°C = Data in relation with process safety (self-ignition temperatures under specific conditions, …) 3 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: data main requirements (3) = Information has to be presented as: - tables - graphs - phase diagrams = Some formulas are necessary: - Q = m c ΔT - Fourier equation 4 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: industrial associations in Europe = = = = 5 Caobisco, Brussels, Belgium ASIC, Paris, France Biscuit, Cake, Chocolate & Confectionery, London, UK ILSI (Int. Life Sciences Institute) Europe, Brussels, Belgium Campden and Chorleywood Food Research Association, Chipping Campden, UK Leatherhead Food International, Leatherhead, UK IVLV ( Industrievereinigung für Lebensmitteltechnologie und Verpackung) Munich, Germany PIRA, Packaging Research, Leatherhead, UK 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: trade publications = Commercial publications such as: - Lebensmittel Technologie (CH) - Der Lebensmittelbrief (GE) - Lebensmittel-Industrie (CH) = Bulletins from INRA, ENSIA, Leatherhead, FSTA, Campden and Chorleywood = Scientific journals such as: - J. of Food Engineering - Science des Aliments 6 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: contacts outside = Anita Mikkonen, VTT Biotekniikka, Finland = Philip Barlow, National University of Singapore, Singapore = Douglas Goff, University of Guelf, Canada = Lebert Grierson, The University of the West Indies, Trinidad and Tobago 7 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: network of contacts = Michel Ollivon, CNRS Chatenay-Malabry, France = Perla Relkin, ENSIA, Massy, France = Yrjö Roos, University College Cork, Ireland = Alberto Schiraldi, DISTAM, Milan, Italy = Danièle Clausse, UT Compiègne, France = Bertrand Roduit, AKTS, Sierre, Switzerland 8 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: food businesses = Coffee: Nestlé, Kraft, Procter and Gamble, … = Oil and fat: Unilever = Tea: Lipton, Nestlé = Dairy: Danone, Nestlé, Milupa = Fish: Findus = Additives, emulsifiers: Danisco, … 9 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: special remarks = It is difficult to have scientific contacts with small food companies = To distribute thermal information in small companies, the best persons would be: - Pierre Le Parlouër, Thermal Consulting, Caluire (F) - dedicated small companies - interested university professors 10 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

First approach: Specific heat (Cp) of foods -Theoretical calculations - Experimental determination - Literature 2004 -01 -15 NRC/FS - ARY Nestlé Research Center
![Evitherm 2004 Specific heat General formula is Qmc T Q J or Evitherm 2004: Specific heat = General formula is: Q=mc T [Q] = J or](https://slidetodoc.com/presentation_image_h2/4d15e5c0f4e831fbecc667dfcaf70b1c/image-12.jpg)
Evitherm 2004: Specific heat = General formula is: Q=mc T [Q] = J or W s [m] = g (or kg) [c] = J / g °C (or J/kg °C) T = °C or K 12 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: Specific heat = Theoretical calculation Food composition has to be known. Specific heat at ambient temperature of the food is obtained by proportional addition of the specific heat of each food constituent (water, carbohydrate, lipid, protein, ashes). 13 2004 -01 -15 NRC/FS - ARY Nestlé Research Center
![Evitherm2004 Specific heat Theoretical calculation Specific heat of food constituents 1 For water Evitherm-2004: Specific heat Theoretical calculation Specific heat of food constituents [1] - For water:](https://slidetodoc.com/presentation_image_h2/4d15e5c0f4e831fbecc667dfcaf70b1c/image-14.jpg)
Evitherm-2004: Specific heat Theoretical calculation Specific heat of food constituents [1] - For water: 4. 18 J/g - For carbohydrate: 1. 42 J/g - For lipid: 1. 67 J/g (somewhat low) - For protein: 1. 55 J/g - For ashes: 0. 84 J/g - For ice: 2. 11 J/g = [1] V. Gekkas, Transport phenomena of foods and biological materials, CRC Press, Boca Raton, 1992. 14 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: Specific heat = Remarks - Specific heats of solids increase with increasing temperature V. Gekas gives the caracteristic parameters of a quadratic equation describing this effect - Specific heat of water does practically not change between 0°C and 100°C - Specific heat depends strongly on the water content of the considered product - Some non-food specific heat values are also of interest (copper, stainless steel, aluminium, …). 15 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

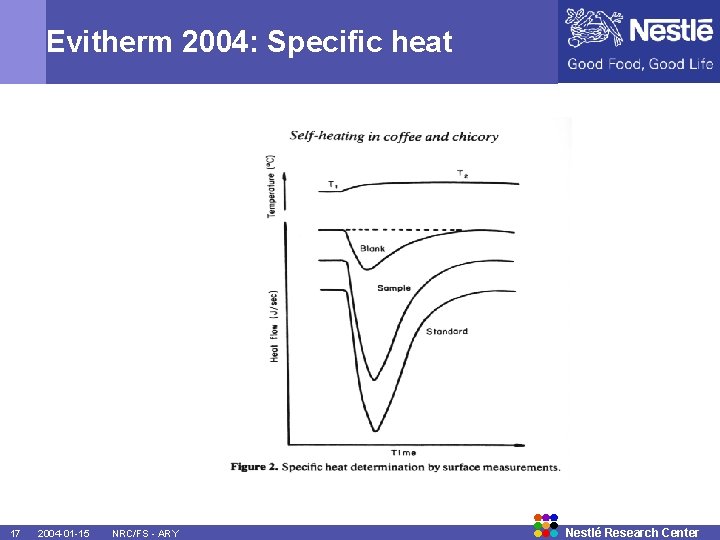
Evitherm 2004: Specific heat = Experimental determination Calorimetric or DSC measurements allow to determine food specific heats at the temperature of interest. = Normally 3 measurements are necessary: - Reference material, usually synthetic sapphire Empty cell Sample cell - 16 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: Specific heat 17 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

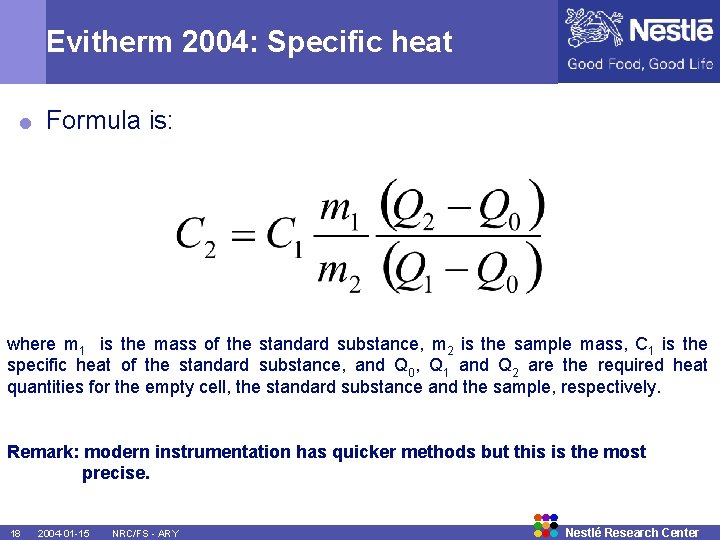
Evitherm 2004: Specific heat = Formula is: where m 1 is the mass of the standard substance, m 2 is the sample mass, C 1 is the specific heat of the standard substance, and Q 0, Q 1 and Q 2 are the required heat quantities for the empty cell, the standard substance and the sample, respectively. Remark: modern instrumentation has quicker methods but this is the most precise. 18 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: Specific heat = Remarks: - Specific heat is generally determined at temperatures where the sample present no phase transitions - Glass transition phenomena are sometimes presented in the specific heat determination - Thermal conductivity is often found in the literature indicating specific heat. 19 2004 -01 -15 NRC/FS - ARY Nestlé Research Center
![Evitherm 2004 Specific heat Literature on specific heat of foods 1 N N Evitherm 2004: Specific heat = Literature on specific heat of foods [1] N. N.](https://slidetodoc.com/presentation_image_h2/4d15e5c0f4e831fbecc667dfcaf70b1c/image-20.jpg)
Evitherm 2004: Specific heat = Literature on specific heat of foods [1] N. N. Mohsenin, Thermal properties of food and agricultural materials, Gordon and Breach, New York, 1980. [2] M. Pyda, Conformational Contribution to the Heat Capacity of the Starch and Water System, J. of Polymer Science: Part B: Polymer Physics, 39 (2001) 3038 -3054. [3] A. Raemy and P. Lambelet, A calorimetric study of selfheating in coffee and chicory, J. Food Technology 17 (1982) 451 -460. 20 2004 -01 -15 NRC/FS - ARY Nestlé Research Center

Evitherm 2004: Specific heat THANK YOU FOR YOUR ATTENTION 21 2004 -01 -15 NRC/FS - ARY Nestlé Research Center
 Conseil psychologique unifr
Conseil psychologique unifr Nutrifreeze
Nutrifreeze Unit 2 food food food
Unit 2 food food food Food chain food chain food chain
Food chain food chain food chain Cdar
Cdar Pearson education, inc
Pearson education, inc Caltech richard murray
Caltech richard murray Tabel rh
Tabel rh T. trimpe 2004 http //sciencespot.net/
T. trimpe 2004 http //sciencespot.net/ Dgc 2004
Dgc 2004 2004 dress code
2004 dress code School grievance procedure in the philippines
School grievance procedure in the philippines Sworn statement for esales registration
Sworn statement for esales registration Legge urbanistica regione campania 16/2004
Legge urbanistica regione campania 16/2004 Kepmenkes no 81 tahun 2004
Kepmenkes no 81 tahun 2004 Perbedaan kurikulum 1994 dengan kurikulum 2004
Perbedaan kurikulum 1994 dengan kurikulum 2004 2004 ford ranger 2.3 firing order
2004 ford ranger 2.3 firing order Age discrimination act 2004
Age discrimination act 2004 Ley idea 2004
Ley idea 2004 Fiona pilkington timeline
Fiona pilkington timeline Lancaster 2004
Lancaster 2004 Anti discrimination act qld
Anti discrimination act qld







































