EVIDENCES OF A CHEMICAL REACTION 1 Color change
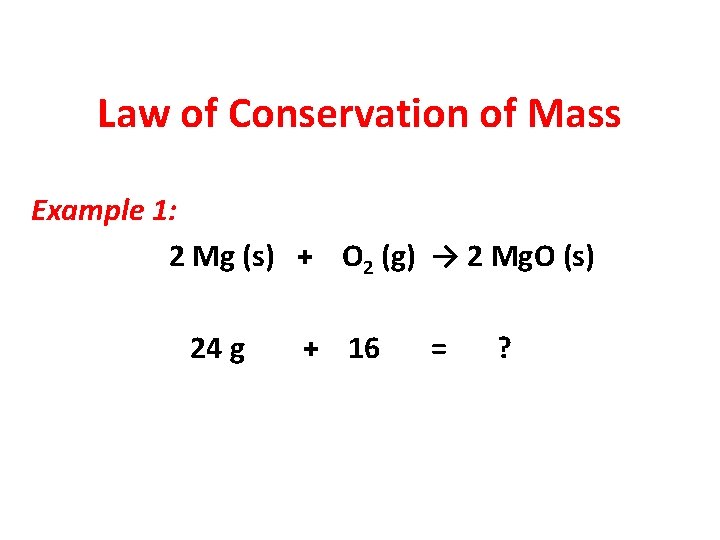
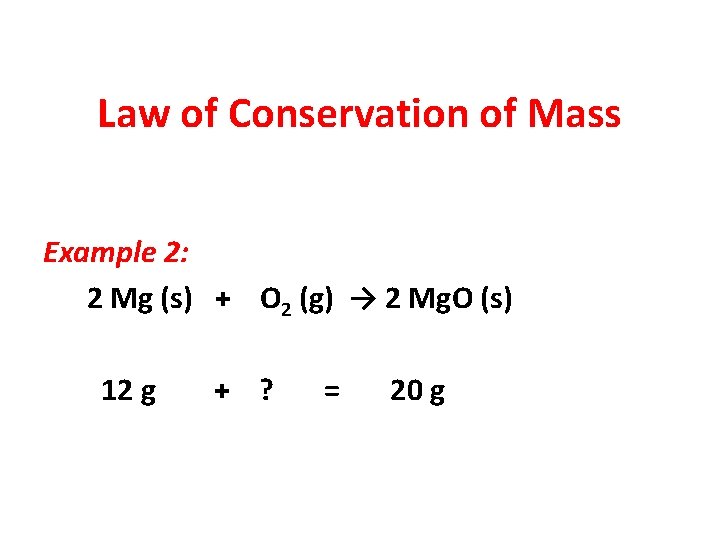
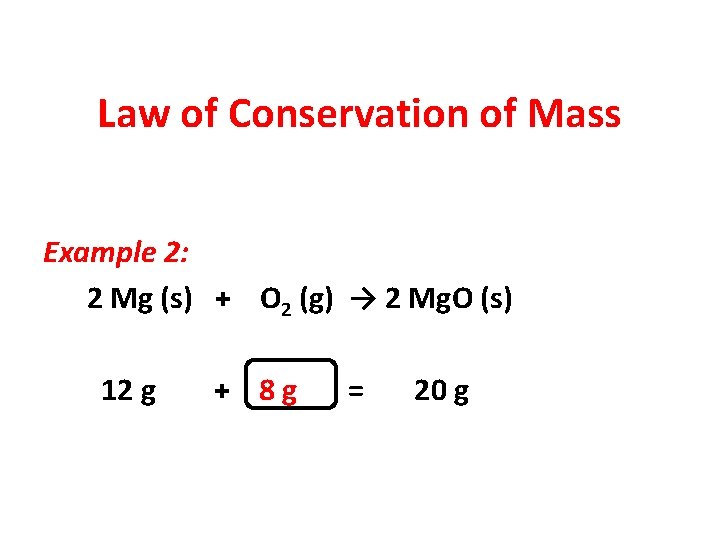
- Slides: 13

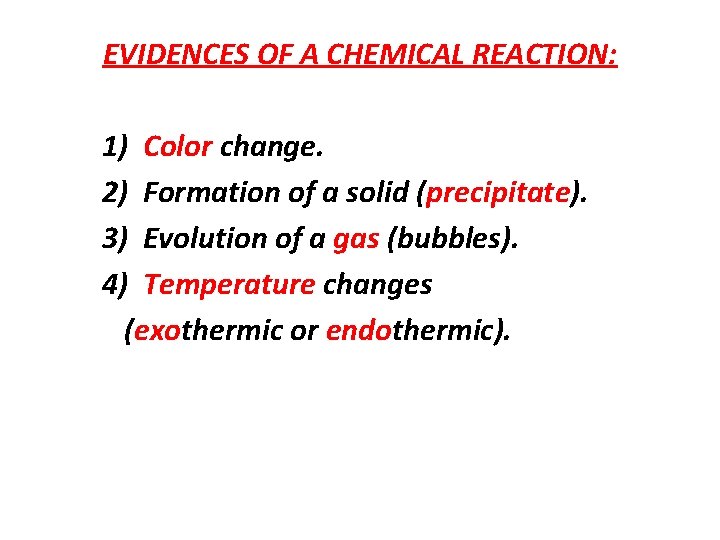
EVIDENCES OF A CHEMICAL REACTION: 1) Color change. 2) Formation of a solid (precipitate). 3) Evolution of a gas (bubbles). 4) Temperature changes (exothermic or endothermic).

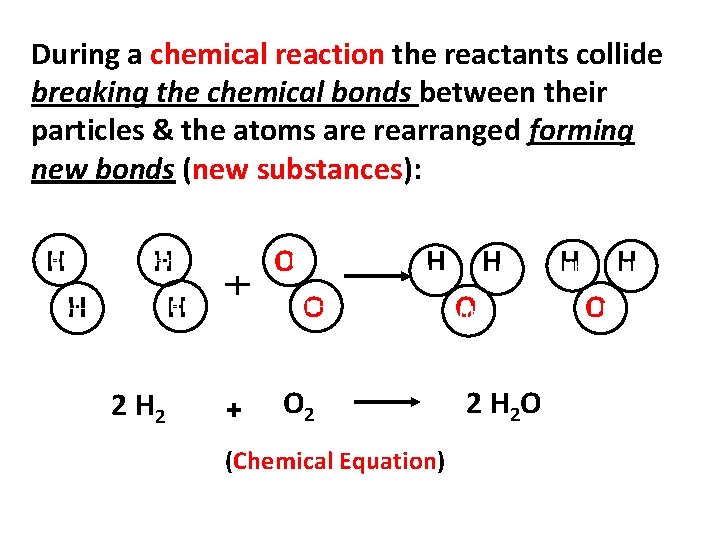
During a chemical reaction the reactants collide breaking the chemical bonds between their particles & the atoms are rearranged forming new bonds (new substances): HH H 2 HH O H HH O + H HH HH O 2 (Chemical Equation) HH O 2 H 2 O H HH HH O

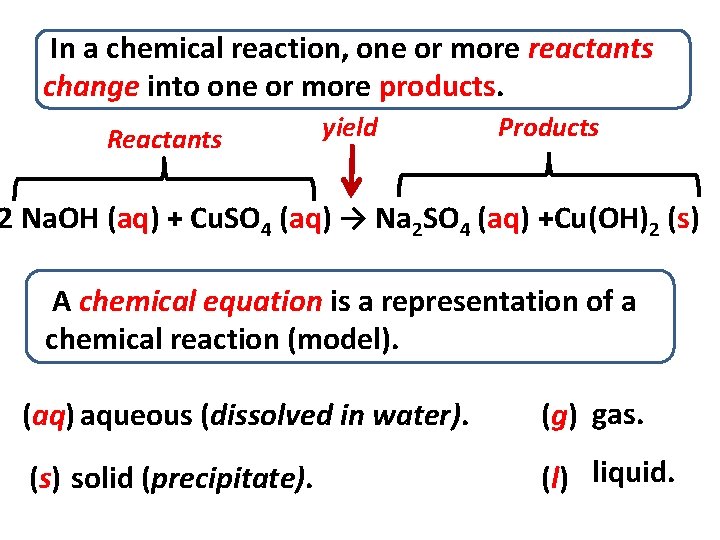
In a chemical reaction, one or more reactants change into one or more products. Reactants yield Products 2 Na. OH (aq) + Cu. SO 4 (aq) → Na 2 SO 4 (aq) +Cu(OH)2 (s) A chemical equation is a representation of a chemical reaction (model). (aq) aqueous (dissolved in water). (g) gas. (s) solid (precipitate). (l) liquid.

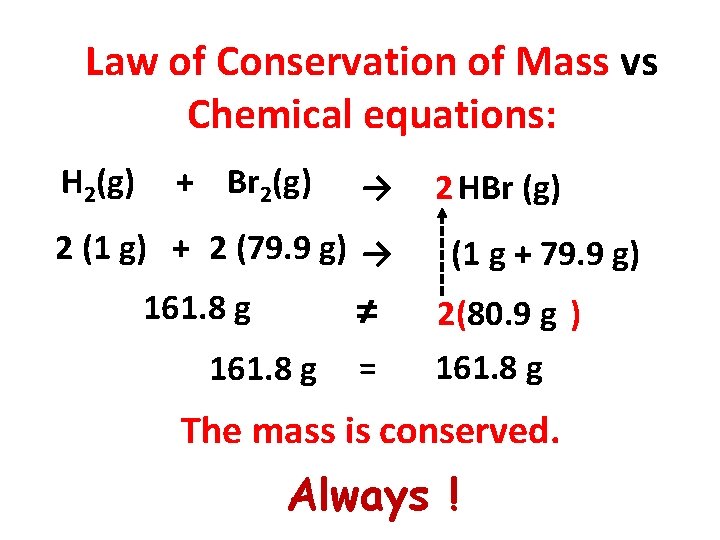
Law of Conservation of Mass vs Chemical equations: H 2(g) + Br 2(g) → 2 (1 g) + 2 (79. 9 g) → 161. 8 g ≠ 161. 8 g = 2 HBr (g) (1 g + 79. 9 g) 2(80. 9 g ) 161. 8 g The mass is conserved. Always !

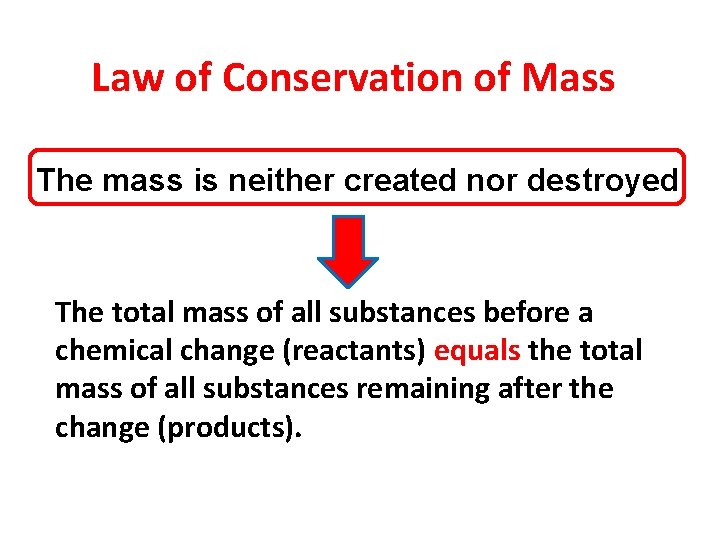
Law of Conservation of Mass The mass is neither created nor destroyed. The total mass of all substances before a chemical change (reactants) equals the total mass of all substances remaining after the change (products).
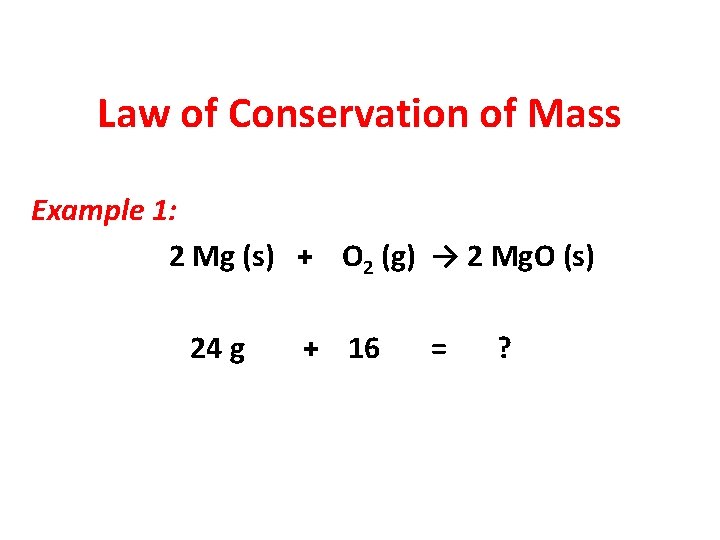
Law of Conservation of Mass Example 1: 2 Mg (s) + O 2 (g) → 2 Mg. O (s) 24 g + 16 = ?

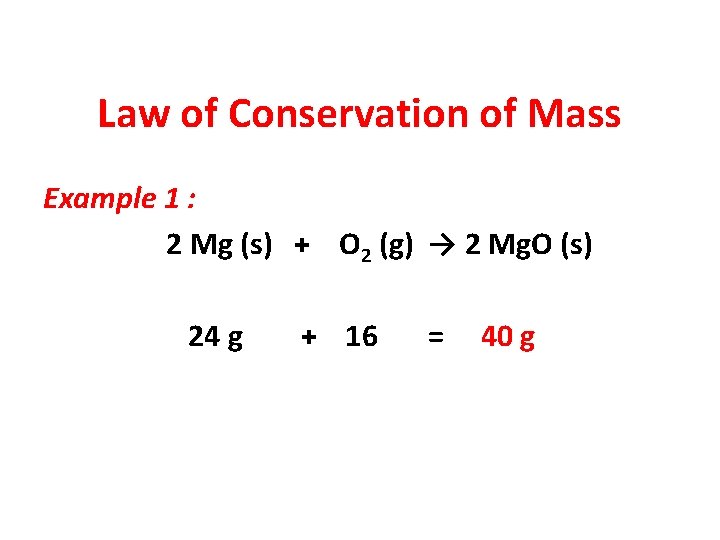
Law of Conservation of Mass Example 1 : 2 Mg (s) + O 2 (g) → 2 Mg. O (s) 24 g + 16 = 40 g
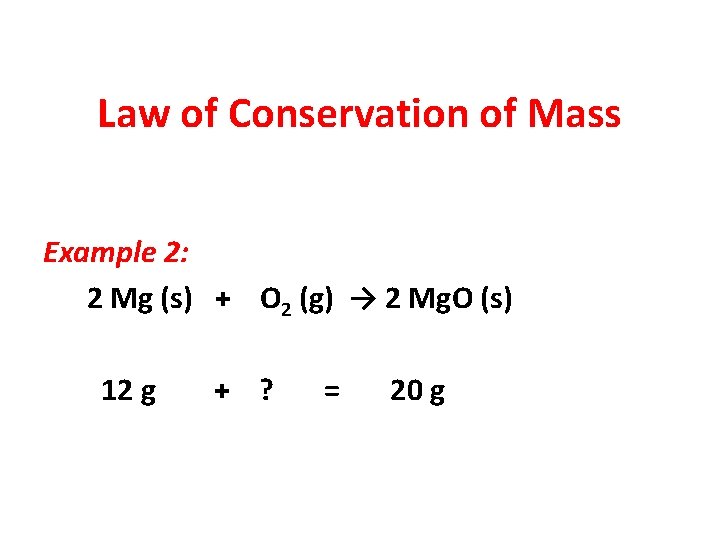
Law of Conservation of Mass Example 2: 2 Mg (s) + O 2 (g) → 2 Mg. O (s) 12 g + ? = 20 g
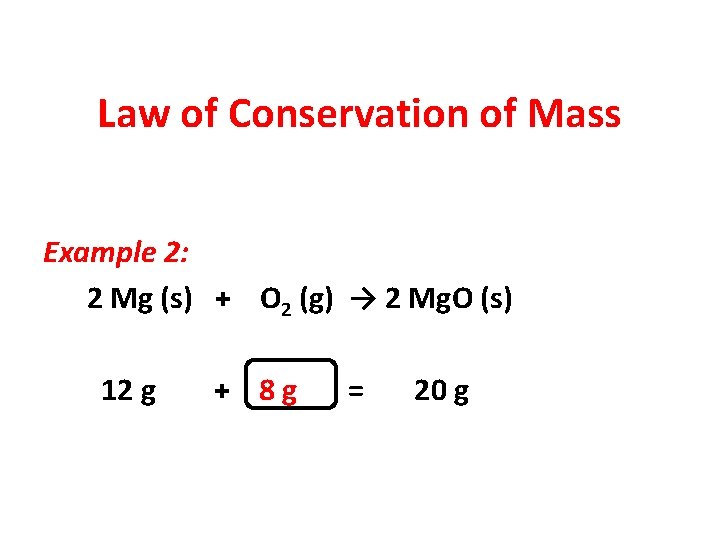
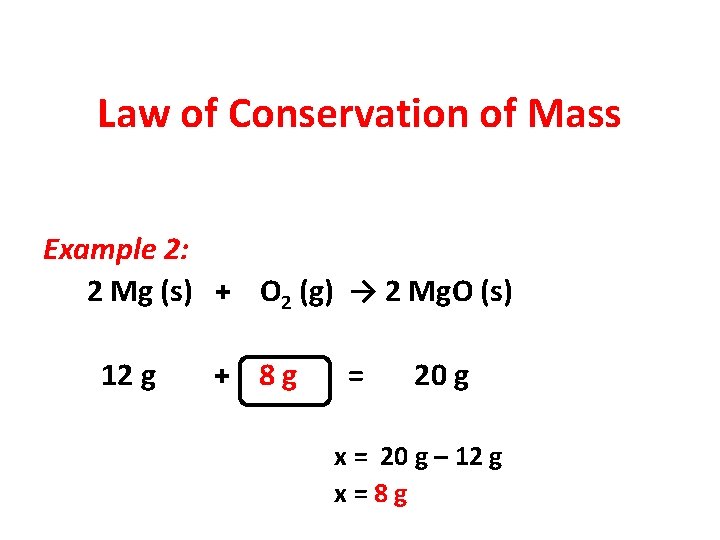
Law of Conservation of Mass Example 2: 2 Mg (s) + O 2 (g) → 2 Mg. O (s) 12 g + 8 g = 20 g

Law of Conservation of Mass Example 2: 2 Mg (s) + O 2 (g) → 2 Mg. O (s) 12 g + 8 g = 20 g x = 20 g – 12 g x=8 g

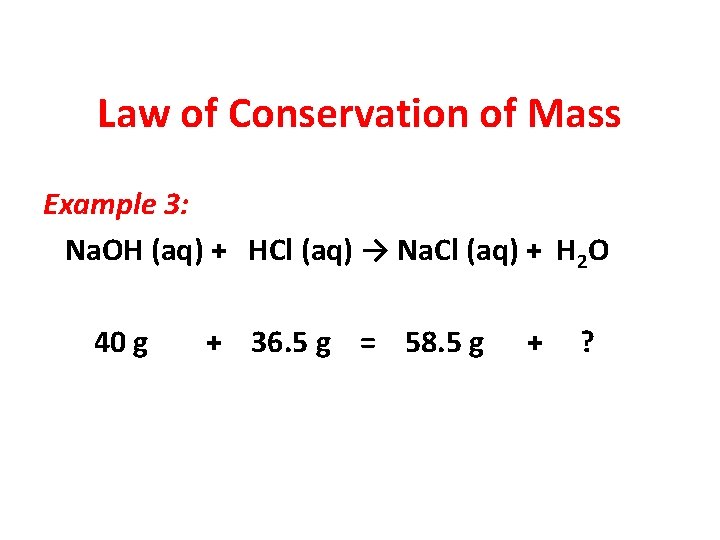
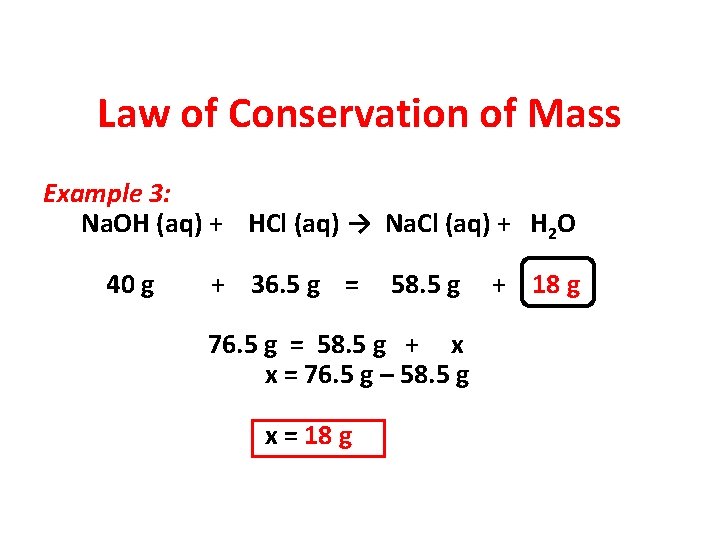
Law of Conservation of Mass Example 3: Na. OH (aq) + HCl (aq) → Na. Cl (aq) + H 2 O 40 g + 36. 5 g = 58. 5 g + ?

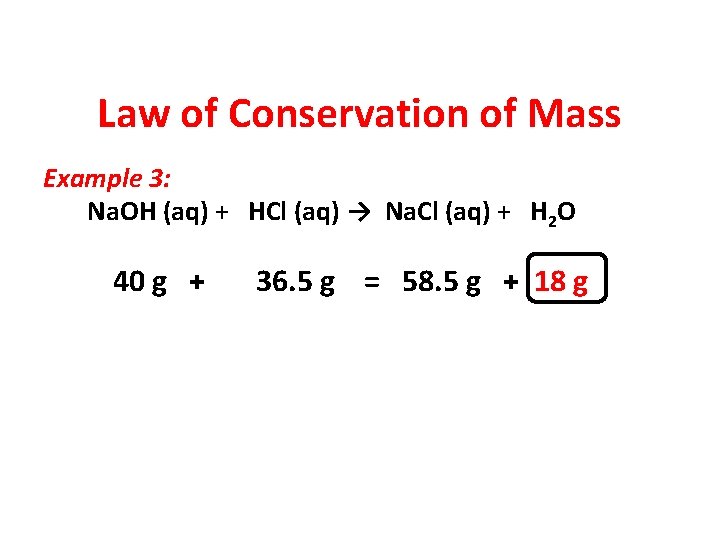
Law of Conservation of Mass Example 3: Na. OH (aq) + HCl (aq) → Na. Cl (aq) + H 2 O 40 g + 36. 5 g = 58. 5 g + 18 g

Law of Conservation of Mass Example 3: Na. OH (aq) + HCl (aq) → Na. Cl (aq) + H 2 O 40 g + 36. 5 g = 58. 5 g 76. 5 g = 58. 5 g + x x = 76. 5 g – 58. 5 g x = 18 g + 18 g