EVIDENCE OF A CHEMICAL REACTION Precipitate Color change


- Slides: 10

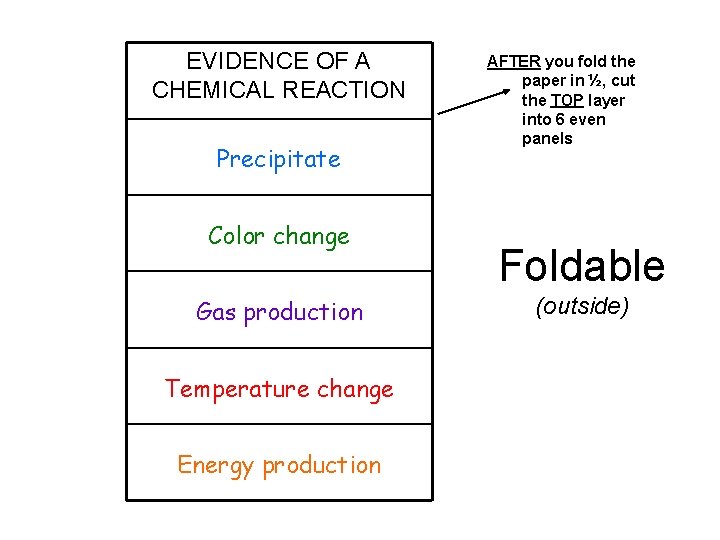
EVIDENCE OF A CHEMICAL REACTION Precipitate Color change Gas production Temperature change Energy production AFTER you fold the paper in ½, cut the TOP layer into 6 even panels Foldable (outside)

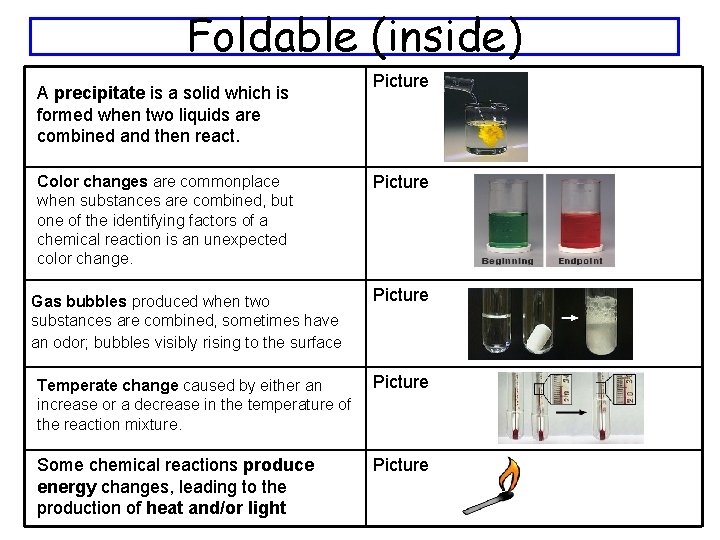
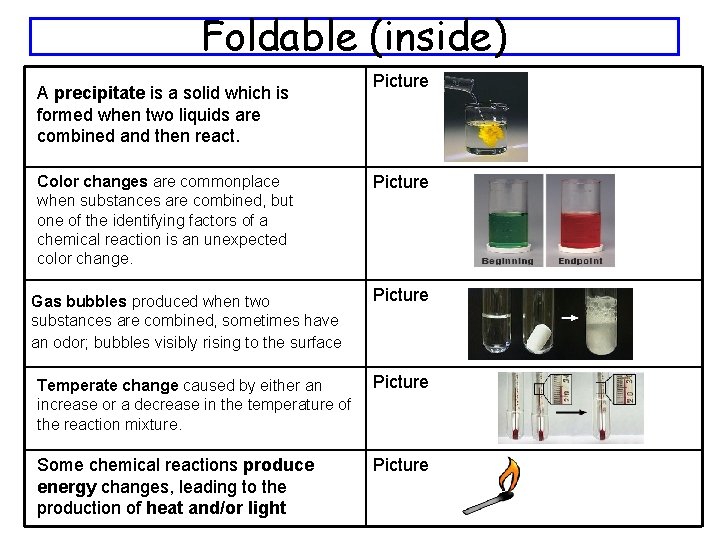
Foldable (inside) A precipitate is a solid which is formed when two liquids are combined and then react. Color changes are commonplace when substances are combined, but one of the identifying factors of a chemical reaction is an unexpected color change. Gas bubbles produced when two substances are combined, sometimes have an odor; bubbles visibly rising to the surface Picture Temperate change caused by either an increase or a decrease in the temperature of the reaction mixture. Picture Some chemical reactions produce energy changes, leading to the production of heat and/or light Picture

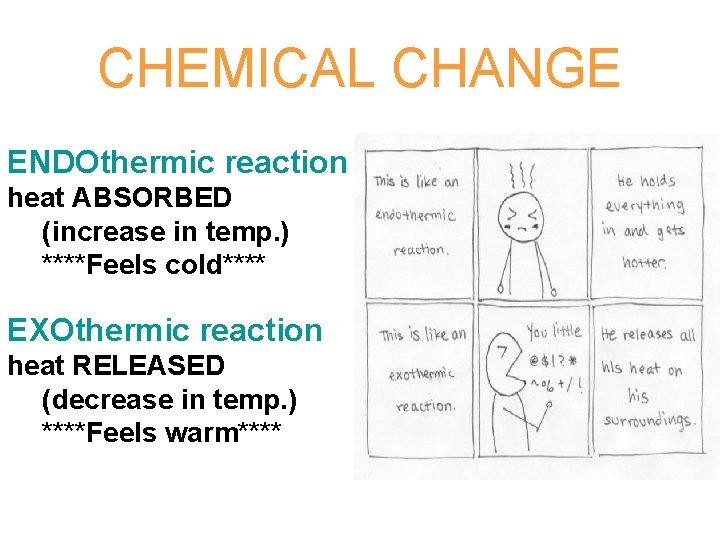
CHEMICAL CHANGE ENDOthermic reaction heat ABSORBED (increase in temp. ) ****Feels cold**** EXOthermic reaction heat RELEASED (decrease in temp. ) ****Feels warm****

Foldable (outside)

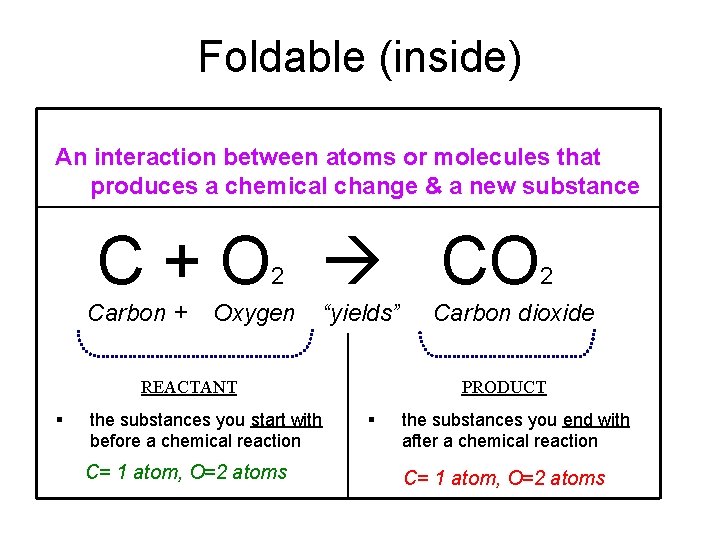
Foldable (inside) An interaction between atoms or molecules that produces a chemical change & a new substance C + O CO 2 Carbon + Oxygen “yields” REACTANT § the substances you start with before a chemical reaction C= 1 atom, O=2 atoms 2 Carbon dioxide PRODUCT § the substances you end with after a chemical reaction C= 1 atom, O=2 atoms

Pour about an inch of liquid--half vinegar, half water--into the bottle. Use the funnel or straw to fill the balloon half full of baking soda. A bottle with a narrow neck • Vinegar • Baking soda • Funnel or straw • Water • Balloon Stretch the open end of the balloon over the neck of the bottle. Make sure it's on tight! Let the heavy end of the balloon dangle, so no baking soda goes in the bottle. Hold onto the balloon at the bottle neck, and pick up the heavy part of the balloon so that all the baking soda falls into the vinegar at the bottom of the bottle. OBSERVE!

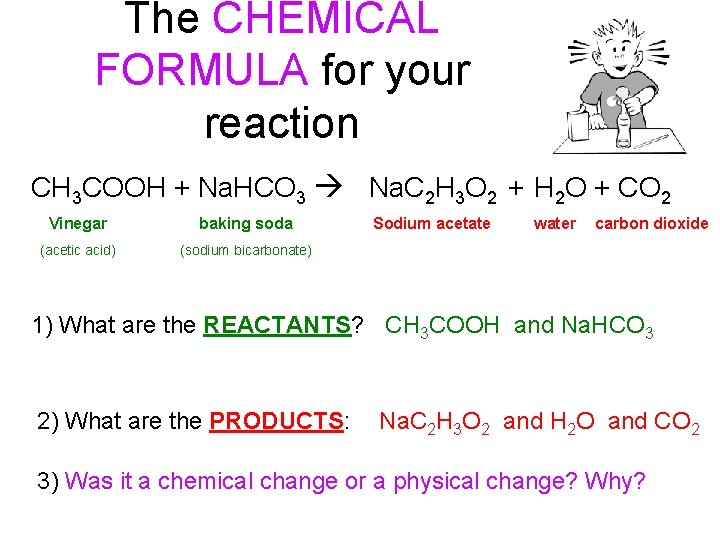
The CHEMICAL FORMULA for your reaction CH 3 COOH + Na. HCO 3 Na. C 2 H 3 O 2 + H 2 O + CO 2 Vinegar baking soda (acetic acid) (sodium bicarbonate) Sodium acetate water carbon dioxide 1) What are the REACTANTS? CH 3 COOH and Na. HCO 3 2) What are the PRODUCTS: Na. C 2 H 3 O 2 and H 2 O and CO 2 3) Was it a chemical change or a physical change? Why?

1) What is the difference between a chemical property and a physical property 2) What is a chemical change? Give an example. 3) What is a physical change? Give an example. 4) Which subatomic particle determines the reactivity of an element 5) Compare & contrast an element, compound, and mixture 6) Compare a metal to a non-metal. 7) Why are Halogens reactive? 8) What are the parts of a chemical reaction? Define each. 9) Find the mass of 265 m. L of benzene. The density of benzene is. 8765 g/m. L 10) A flask that weighs 452. 6 g is filled with 215 m. L of sodium bicarbonate. The weight of the flask & sodium bicarbonate is 752. 5 g. Calculate the density of sodium bicarbonate.

EVIDENCE OF A CHEMICAL REACTION Precipitate Color change Gas production Temperature change Energy production Foldable (outside)

Foldable (inside) A precipitate is a solid which is formed when two liquids are combined and then react. Color changes are commonplace when substances are combined, but one of the identifying factors of a chemical reaction is an unexpected color change. Gas bubbles produced when two substances are combined, sometimes have an odor; bubbles visibly rising to the surface Picture Temperate change caused by either an increase or a decrease in the temperature of the reaction mixture. Picture Some chemical reactions produce energy changes, leading to the production of heat and/or light Picture