Evidence for Atoms and Subatomic Particles From Democritus

Evidence for Atoms and Subatomic Particles

From Democritus to Dalton • 400 BC a Greek philosopher named Democritus proposed that everything is made of “atomos. ” His idea was less popular than Aristotle’s 4 elements (earth, wind, fire, water). • 1800 AD John Dalton, an English scientist, proposed the first Atomic Theory: 1. Elements are made of tiny particles called atoms 2. All atoms of a given element are identical 3. Atoms of one element are different from atoms of another element 4. Atoms cannot be created or destroyed

JJ Thomson • 1897 - Thomson was a professor in England • Experimented with cathode rays • Found that they were negatively charged and made up of particles • Named the particles “electrons”

Thomson’s Apparatus • A cathode ray tube is a vacuum tube made of glass. • When connected to a power source, a green ray appears inside the glass. • The ray is deflected by a magnet (showing that it is charged). • The ray can move a paddle wheel along a track (showing it is made up of particles).
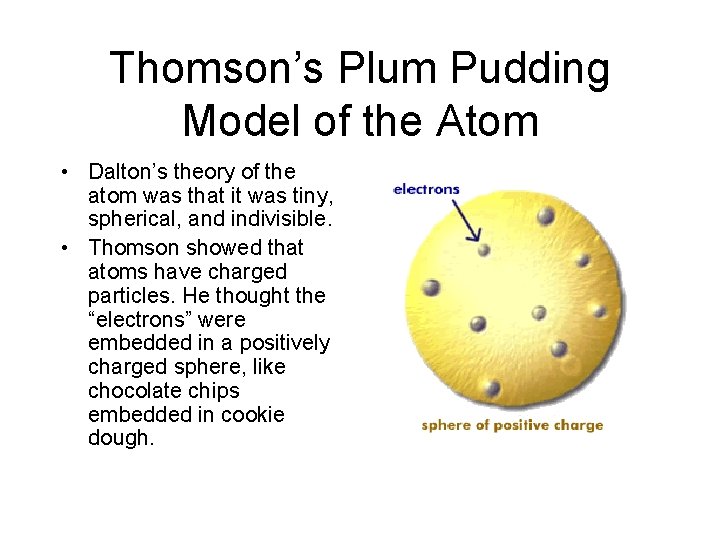
Thomson’s Plum Pudding Model of the Atom • Dalton’s theory of the atom was that it was tiny, spherical, and indivisible. • Thomson showed that atoms have charged particles. He thought the “electrons” were embedded in a positively charged sphere, like chocolate chips embedded in cookie dough.

Rutherford’s Experiment • Ernest Rutherford studied under Thomson. • He was not satisfied with the Plum Pudding model because he thought it showed that the atom held the electrons too tightly. • Rutherford showed that the positive charge in the atom is concentrated in the center of the atom…which he called the nucleus.
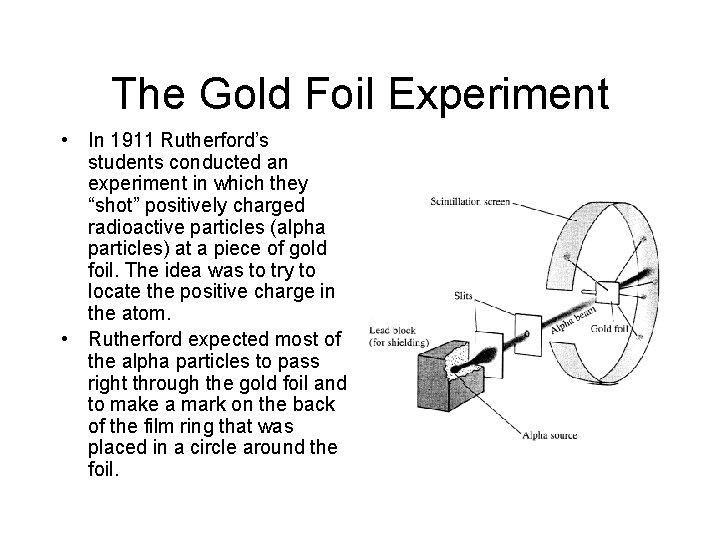
The Gold Foil Experiment • In 1911 Rutherford’s students conducted an experiment in which they “shot” positively charged radioactive particles (alpha particles) at a piece of gold foil. The idea was to try to locate the positive charge in the atom. • Rutherford expected most of the alpha particles to pass right through the gold foil and to make a mark on the back of the film ring that was placed in a circle around the foil.

Rutherford’s Conclusion • Most of the alpha particles did pass right through the foil, but a few were deflected. • The deflections demonstrated that most of the atom is empty space but that the positive charge in the nucleus was in a very small, but very dense area. • Rutherford called this area the “nucleus”.
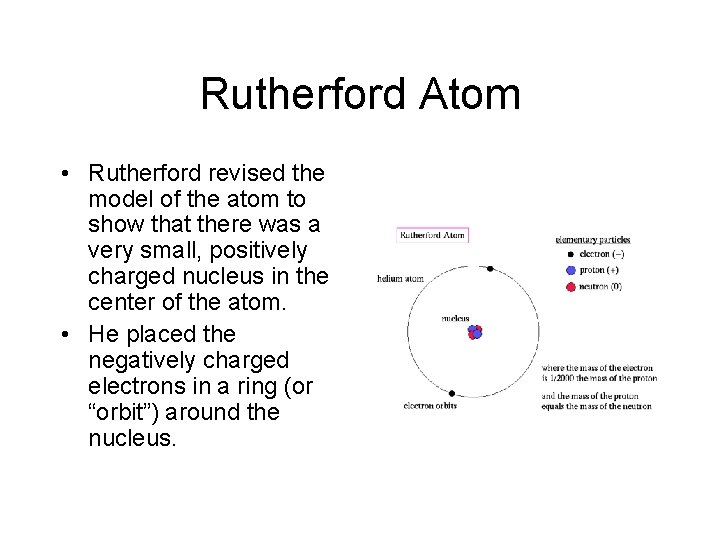
Rutherford Atom • Rutherford revised the model of the atom to show that there was a very small, positively charged nucleus in the center of the atom. • He placed the negatively charged electrons in a ring (or “orbit”) around the nucleus.

Niels Bohr and the Electron Orbitals • 1914 - Niels Bohr was trying to explain the energy given off by gases that were heated by electricity. • Bohr showed that electrons do not all share the same energy level. There is more than one possible “orbit” around the nucleus.
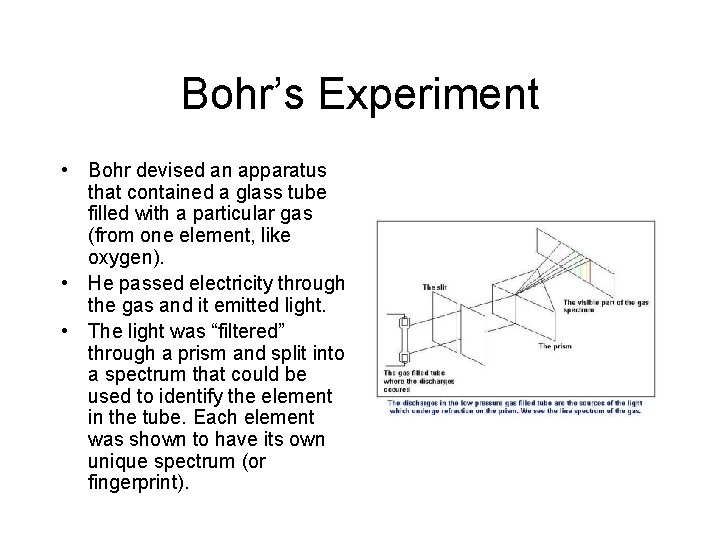
Bohr’s Experiment • Bohr devised an apparatus that contained a glass tube filled with a particular gas (from one element, like oxygen). • He passed electricity through the gas and it emitted light. • The light was “filtered” through a prism and split into a spectrum that could be used to identify the element in the tube. Each element was shown to have its own unique spectrum (or fingerprint).
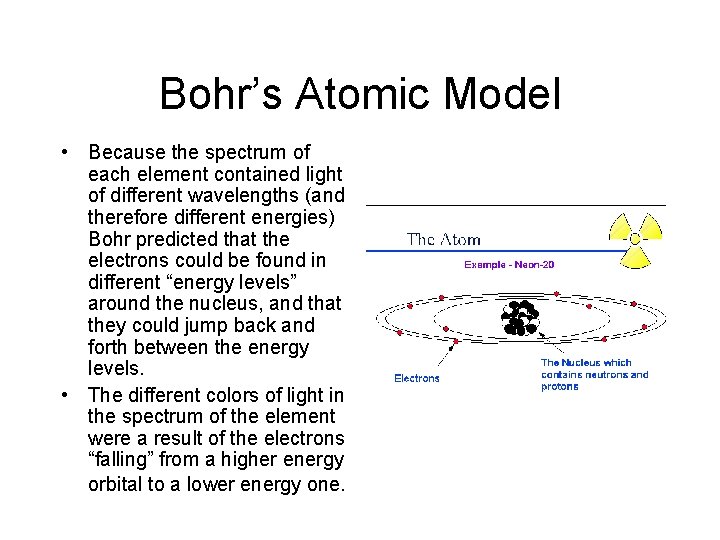
Bohr’s Atomic Model • Because the spectrum of each element contained light of different wavelengths (and therefore different energies) Bohr predicted that the electrons could be found in different “energy levels” around the nucleus, and that they could jump back and forth between the energy levels. • The different colors of light in the spectrum of the element were a result of the electrons “falling” from a higher energy orbital to a lower energy one.
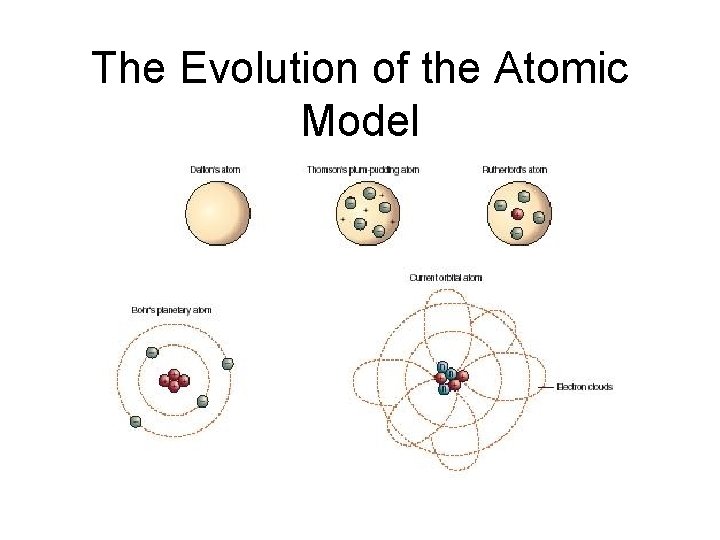
The Evolution of the Atomic Model
- Slides: 13